Exam 2: Basic Chemistry
Exam 1: A View of Life58 Questions
Exam 2: Basic Chemistry58 Questions
Exam 3: The Chemistry of Organic Molecules55 Questions
Exam 4: Cell Structure and Function58 Questions
Exam 5: Membrane Structure and Function60 Questions
Exam 6: Metabolism: Energy and Enzymes52 Questions
Exam 7: Photosynthesis55 Questions
Exam 8: Cellular Respiration56 Questions
Exam 9: The Cell Cycle and Cellular Reproduction56 Questions
Exam 10: Meiosis and Sexual Reproduction62 Questions
Exam 11: Mendelian Patterns of Inheritance63 Questions
Exam 12: Molecular Biology of the Gene49 Questions
Exam 13: Regulation of Gene Expression51 Questions
Exam 14: Biotechnology and Genomics51 Questions
Exam 15: Darwin and Evolution60 Questions
Exam 16: How Populations Evolve56 Questions
Exam 17: Speciation and Macroevolution57 Questions
Exam 18: Origin and History of Life57 Questions
Exam 19: Taxonomy, Systematics, and Phylogeny56 Questions
Exam 20: Viruses, Bacteria, and Archaea51 Questions
Exam 21: Protist Evolution and Diversity50 Questions
Exam 22: Fungi Evolution and Diversity58 Questions
Exam 23: Plant Evolution and Diversity58 Questions
Exam 24: Flowering Plants: Structureand Organization59 Questions
Exam 25: Flowering Plants: Nutrition and Transport56 Questions
Exam 26: Flowering Plants: Control of Growth Responses52 Questions
Exam 27: Flowering Plants: Reproduction53 Questions
Exam 28: Invertebrate Evolution54 Questions
Exam 29: Vertebrate Evolution56 Questions
Exam 30: Human Evolution52 Questions
Exam 31: Animal Organization and Homeostasis52 Questions
Exam 32: Circulation and Cardiovascular Systems57 Questions
Exam 33: The Lymphatic and Immune Systems57 Questions
Exam 34: Digestive Systems and Nutrition55 Questions
Exam 35: Respiratory Systems51 Questions
Exam 36: Body Fluid Regulation and Excretory Systems50 Questions
Exam 37: Neurons and Nervous Systems54 Questions
Exam 38: Sense Organs56 Questions
Exam 39: Locomotion and Support Systems51 Questions
Exam 40: Hormones and Endocrine Systems51 Questions
Exam 41: Reproductive Systems58 Questions
Exam 42: Animal Development54 Questions
Exam 43: Behavioral Ecology51 Questions
Exam 44: Population Ecology53 Questions
Exam 45: Community and Ecosystem Ecology53 Questions
Exam 46: Major Ecosystems of the Biosphere57 Questions
Exam 47: Conservation of Biodiversity50 Questions
Select questions type
All of the following are examples of damage caused by acid deposition from rain EXCEPT
Free
(Multiple Choice)
4.7/5  (35)
(35)
Correct Answer:
C
Human blood has a pH of about 7.4. This is
Free
(Multiple Choice)
4.8/5  (43)
(43)
Correct Answer:
D
The characteristic way in which atoms of an element react is most related to the
Free
(Multiple Choice)
4.8/5  (31)
(31)
Correct Answer:
A
A solution with a pH of 6 has ________ times _________ OH- than a solution with a pH of 10.
(Multiple Choice)
4.9/5  (46)
(46)
If an element contains 8 electrons how many electrons will be placed in the 2nd valence shell?
(Multiple Choice)
4.8/5  (34)
(34)
Study the figures to determine which is liquid water and which is frozen water (ice). Explain your answer and predict if the water in Figure 2 would float or sink in the water in Figure 1. 
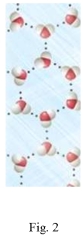
(Essay)
4.8/5  (33)
(33)
A solution with a pH of 7.0 has _______ times ________ H+ than a solution of pH 10.
(Multiple Choice)
4.9/5  (46)
(46)
Draw the structural formula of a single water molecule. Note the location of partial positive and negative charges. Label the covalent bonds. 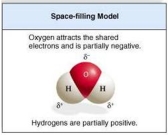
(Essay)
4.9/5  (48)
(48)
Which of the following would be a proposed mechanism by which stomach antacids work?
(Multiple Choice)
4.8/5  (39)
(39)
Draw three water molecules and the hydrogen bonding that may occur between the molecules. Define hydrogen bonding and explain how and why it occurs. 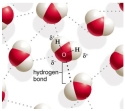
(Essay)
4.9/5  (40)
(40)
What is the maximum number of electrons that will be in the 1st valence shell?
(Multiple Choice)
5.0/5  (37)
(37)
A change of one pH unit represents a ten-fold increase or decrease in hydroxyl ion concentration.
(True/False)
4.7/5  (39)
(39)
Which property of water allows it to act as a transport medium?
(Multiple Choice)
4.7/5  (28)
(28)
 From the above table of radioisotopes and their properties, it is obvious that
From the above table of radioisotopes and their properties, it is obvious that
(Multiple Choice)
4.8/5  (31)
(31)
Showing 1 - 20 of 58
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)