Exam 2: Basic Chemistry
Exam 1: A View of Life58 Questions
Exam 2: Basic Chemistry58 Questions
Exam 3: The Chemistry of Organic Molecules55 Questions
Exam 4: Cell Structure and Function58 Questions
Exam 5: Membrane Structure and Function60 Questions
Exam 6: Metabolism: Energy and Enzymes52 Questions
Exam 7: Photosynthesis55 Questions
Exam 8: Cellular Respiration56 Questions
Exam 9: The Cell Cycle and Cellular Reproduction56 Questions
Exam 10: Meiosis and Sexual Reproduction62 Questions
Exam 11: Mendelian Patterns of Inheritance63 Questions
Exam 12: Molecular Biology of the Gene49 Questions
Exam 13: Regulation of Gene Expression51 Questions
Exam 14: Biotechnology and Genomics51 Questions
Exam 15: Darwin and Evolution60 Questions
Exam 16: How Populations Evolve56 Questions
Exam 17: Speciation and Macroevolution57 Questions
Exam 18: Origin and History of Life57 Questions
Exam 19: Taxonomy, Systematics, and Phylogeny56 Questions
Exam 20: Viruses, Bacteria, and Archaea51 Questions
Exam 21: Protist Evolution and Diversity50 Questions
Exam 22: Fungi Evolution and Diversity58 Questions
Exam 23: Plant Evolution and Diversity58 Questions
Exam 24: Flowering Plants: Structureand Organization59 Questions
Exam 25: Flowering Plants: Nutrition and Transport56 Questions
Exam 26: Flowering Plants: Control of Growth Responses52 Questions
Exam 27: Flowering Plants: Reproduction53 Questions
Exam 28: Invertebrate Evolution54 Questions
Exam 29: Vertebrate Evolution56 Questions
Exam 30: Human Evolution52 Questions
Exam 31: Animal Organization and Homeostasis52 Questions
Exam 32: Circulation and Cardiovascular Systems57 Questions
Exam 33: The Lymphatic and Immune Systems57 Questions
Exam 34: Digestive Systems and Nutrition55 Questions
Exam 35: Respiratory Systems51 Questions
Exam 36: Body Fluid Regulation and Excretory Systems50 Questions
Exam 37: Neurons and Nervous Systems54 Questions
Exam 38: Sense Organs56 Questions
Exam 39: Locomotion and Support Systems51 Questions
Exam 40: Hormones and Endocrine Systems51 Questions
Exam 41: Reproductive Systems58 Questions
Exam 42: Animal Development54 Questions
Exam 43: Behavioral Ecology51 Questions
Exam 44: Population Ecology53 Questions
Exam 45: Community and Ecosystem Ecology53 Questions
Exam 46: Major Ecosystems of the Biosphere57 Questions
Exam 47: Conservation of Biodiversity50 Questions
Select questions type
A research article indicates that researchers have used an isotope 3H to trace a certain metabolic process. From the symbol that is given, we know this is a hydrogen isotope with
(Multiple Choice)
4.9/5  (38)
(38)
The mass number refers to the number of ______ and ______ within an element.
(Multiple Choice)
4.9/5  (37)
(37)
Following nitrogen (78%) and oxygen (21%), argon is the next most common gas in the atmosphere (less than 1%). Checking the table of elements, you discover that argon is one of a family of atoms with outer shells already full of electrons. How is this related to the fact that these atoms have virtually no biological importance?
(Essay)
4.8/5  (37)
(37)
What does this graph reveal about the heat of vaporization and the heat of fusion?
(Essay)
4.9/5  (38)
(38)
This system of chemicals, 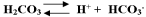 , act as a buffer in the blood. If hydrogen ions are added to blood which of the following reactions would occur?
, act as a buffer in the blood. If hydrogen ions are added to blood which of the following reactions would occur?
(Multiple Choice)
4.8/5  (47)
(47)
If the atomic number of an element is 6 and the atomic mass is 12.01, how many protons are there in the nucleus?
(Multiple Choice)
4.8/5  (32)
(32)
Draw two hydrogen atoms using Bohr's model. Now bond them to form a molecule of hydrogen gas. Write the molecular formula. Explain what type of bond you've created and why this is a stable situation. 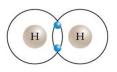
(Essay)
4.7/5  (45)
(45)
Which of the following elements would be the most reactive with other elements?
(Multiple Choice)
4.9/5  (31)
(31)
Both 18O and 16O are found in nature. However, 16O is the most common. Therefore,
(Multiple Choice)
4.8/5  (36)
(36)
The scale indicates the relative concentrations of hydrogen and hydroxyl ions in a solution.
(True/False)
4.9/5  (30)
(30)
Use Bohr's model to draw a sodium (Na) atom and a chlorine (Cl) atom. Using your model, explain what happens when sodium reacts with chlorine to form table salt. Include in your explanation ion and ionic bond formation. Use your model to help you to decide whether NaCl is hydrophilic or hydrophobic. 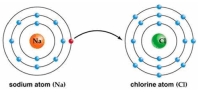
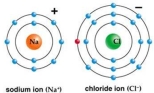
(Essay)
4.8/5  (38)
(38)
Showing 41 - 58 of 58
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)