Exam 5: Aromatic Compounds
Exam 1: Structure and Bonding:acids and Bases40 Questions
Exam 2: Alkanes: The Nature of Organic Compounds47 Questions
Exam 3: Alkenes and Alkynes: the Nature of Organic Reactions37 Questions
Exam 4: Reactions of Alkenes and Alkynes44 Questions
Exam 5: Aromatic Compounds51 Questions
Exam 6: Sterechemistry at Tetrahedral Centers35 Questions
Exam 7: Organohalides: Nucleophilic Substitutions and Eliminations49 Questions
Exam 8: Alcohols, Phenols, Ethers, and Their Sulfur Analogs39 Questions
Exam 9: Aldehydes and Ketones: Nucleophilic Addition Reactions32 Questions
Exam 10: Carboxylic Acids and Derivatives: Nucleophilic Acyl Substitution Reactions66 Questions
Exam 11: Carbonyl Alpha-Substitution Reactions and Condensation Reactions37 Questions
Exam 12: Amines31 Questions
Exam 13: Structure Determination64 Questions
Exam 14: Biomolecules: Carbohydrates41 Questions
Exam 15: Biomolecules: Amino Acids, Peptides, and Proteins43 Questions
Exam 16: Biomolecules: Lipids and Nucleic Acids37 Questions
Exam 17: The Organic Chemistry of Metabolic Pathways35 Questions
Select questions type
Instructions:
Given the major organic product(s) of each of the following reactions. If no reaction is predicted, write "N.R."
Write the product: 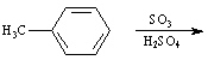
Free
(Essay)
4.7/5  (34)
(34)
Correct Answer:
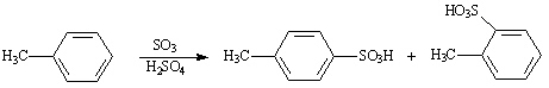
Instructions: Provide the IUPAC name for each of the following compounds.
IUPAC Name:
Free
(Essay)
4.8/5  (35)
(35)
Correct Answer:
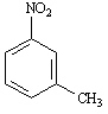 m-nitrotoluene
m-nitrotoluene
What is the major organic product obtained from the following reaction? 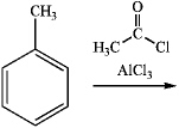
Free
(Multiple Choice)
4.8/5  (38)
(38)
Correct Answer:
C
Instructions: Consider the reaction below to answer the following question(s). 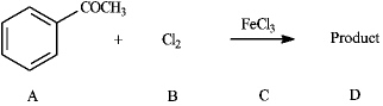 Refer to instructions. This reaction proceeds _____________ (faster or slower) than benzene.
Refer to instructions. This reaction proceeds _____________ (faster or slower) than benzene.
(Short Answer)
4.8/5  (47)
(47)
Which of the following sets of substituents are all o/p-directing in electrophilic aromatic substitution reactions?
(Multiple Choice)
4.8/5  (41)
(41)
Instructions: Consider the reaction below to answer the following question(s). 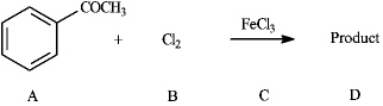 Refer to instructions. Draw the structure of product
D.
Refer to instructions. Draw the structure of product
D.
(Essay)
4.8/5  (36)
(36)
Instructions:
Consider the Friedel-Crafts alkylation reaction below to answer the following questions: 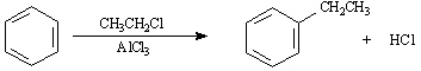 What is the role of the AlCl3 in the reaction?
What is the role of the AlCl3 in the reaction?
(Essay)
4.8/5  (33)
(33)
Consider the production of 1-bromo-4-nitrobenzene: 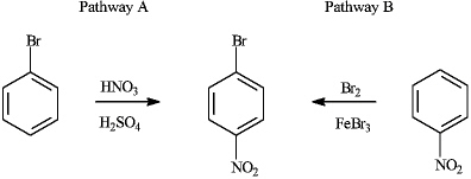 a) Explain why Pathway A for the production of 1-bromo-4-nitrobenzene produces a higher yield of the product.
b) If reaction Pathway A is carried out at high temperatures, a trisubstituted product forms. Draw the structure of the product and explain why it forms.
a) Explain why Pathway A for the production of 1-bromo-4-nitrobenzene produces a higher yield of the product.
b) If reaction Pathway A is carried out at high temperatures, a trisubstituted product forms. Draw the structure of the product and explain why it forms.
(Essay)
4.9/5  (41)
(41)
Instructions:
Given the major organic product(s) of each of the following reactions. If no reaction is predicted, write "N.R."
Write the product:
(Essay)
4.9/5  (46)
(46)
What is the correct assignment of the names of the following aromatic compounds? 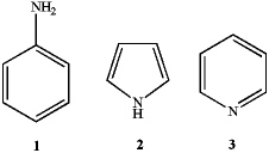
(Multiple Choice)
4.8/5  (37)
(37)
Instructions: Consider the reaction below to answer the following question(s). 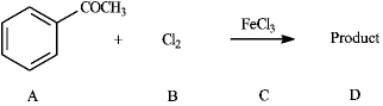 Refer to instructions. The nucleophile in the reaction is indicated by letter _____.
Refer to instructions. The nucleophile in the reaction is indicated by letter _____.
(Short Answer)
4.9/5  (25)
(25)
Circle the compound(s) in the following set that will undergo both substitution and addition when treated with Br2. 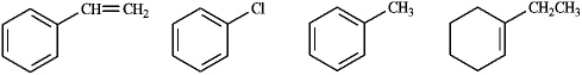
(Essay)
4.8/5  (30)
(30)
Which of the following undergoes the most rapid acylation upon treatment with acetyl chloride and AlCl3?
(Multiple Choice)
4.7/5  (28)
(28)
Instructions: Consider the reaction below to answer the following question(s). 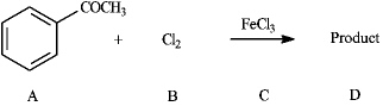 Refer to instructions. The Lewis acid catalyst in the reaction is indicated by letter _____.
Refer to instructions. The Lewis acid catalyst in the reaction is indicated by letter _____.
(Short Answer)
4.7/5  (34)
(34)
Showing 1 - 20 of 51
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)