Exam 11: Carbonyl Alpha-Substitution Reactions and Condensation Reactions
Exam 1: Structure and Bonding:acids and Bases40 Questions
Exam 2: Alkanes: The Nature of Organic Compounds47 Questions
Exam 3: Alkenes and Alkynes: the Nature of Organic Reactions37 Questions
Exam 4: Reactions of Alkenes and Alkynes44 Questions
Exam 5: Aromatic Compounds51 Questions
Exam 6: Sterechemistry at Tetrahedral Centers35 Questions
Exam 7: Organohalides: Nucleophilic Substitutions and Eliminations49 Questions
Exam 8: Alcohols, Phenols, Ethers, and Their Sulfur Analogs39 Questions
Exam 9: Aldehydes and Ketones: Nucleophilic Addition Reactions32 Questions
Exam 10: Carboxylic Acids and Derivatives: Nucleophilic Acyl Substitution Reactions66 Questions
Exam 11: Carbonyl Alpha-Substitution Reactions and Condensation Reactions37 Questions
Exam 12: Amines31 Questions
Exam 13: Structure Determination64 Questions
Exam 14: Biomolecules: Carbohydrates41 Questions
Exam 15: Biomolecules: Amino Acids, Peptides, and Proteins43 Questions
Exam 16: Biomolecules: Lipids and Nucleic Acids37 Questions
Exam 17: The Organic Chemistry of Metabolic Pathways35 Questions
Select questions type
Instructions: Draw the structure of the aldol self-condensation product of each compound for the following question(s). If a compound does not undergo aldol self-condensation, explain why it does not.
Draw and explain:

Free
(Essay)
4.9/5  (36)
(36)
Correct Answer:
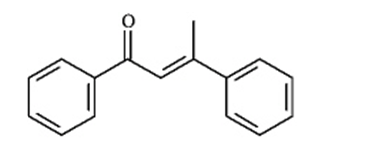
Consider the reaction below to answer the following questions.
 Refer to Instructions. The enolate ion in the reaction is:
Refer to Instructions. The enolate ion in the reaction is:
Free
(Short Answer)
4.8/5  (43)
(43)
Correct Answer:
C
The common feature of a-substitution and condensation reactions of carbonyl groups:
Free
(Multiple Choice)
4.9/5  (32)
(32)
Correct Answer:
B
Instructions: Draw the structure of the product you would expect to obtain by Claisen condensation of the esters shown in the question(s) below. If an ester does not undergo Claisen condensation, explain why it does not.
Draw and explain:
(Short Answer)
4.8/5  (26)
(26)
How would you prepare each of the following compounds using a malonic ester synthesis? Show all intermediate structures and all reagents.
Prepare:
 Chapter 11 - Carbonyl Alpha-Substitution Reactions and Condensation Reactions Key
Chapter 11 - Carbonyl Alpha-Substitution Reactions and Condensation Reactions Key
(Essay)
4.9/5  (33)
(33)
Instructions: Consider the reaction below to answer the following question(s).
 Refer to instructions. The product of this reaction is:
Refer to instructions. The product of this reaction is:
(Multiple Choice)
4.7/5  (34)
(34)
Instructions: Refer to the compounds below to answer the following question(s).  Refer to instructions. Draw the structure for the enol and enolate ions corresponding to Compound I.
Refer to instructions. Draw the structure for the enol and enolate ions corresponding to Compound I.
(Essay)
4.7/5  (35)
(35)
Instructions: Each of the following compounds in the following question(s) can be prepared by a mixed aldol condensation reaction.
a) Give the structures of the aldehyde and/or ketone precursors for each aldol condensation product and formulate the reaction.
b) Give the structure of the intermediate aldol product.
Refer to instructions. Use the following compound: 
(Essay)
4.9/5  (35)
(35)
Rank the following hydrogens in terms of decreasing acidity (most acidic > least acidic):

(Short Answer)
5.0/5  (43)
(43)
Instructions: Draw the structure of the aldol self-condensation product of each compound for the following question(s). If a compound does not undergo aldol self-condensation, explain why it does not.
Draw and explain:

(Essay)
4.9/5  (28)
(28)
Instructions: Consider the reaction below to answer the following question(s).
 Refer to instructions. Draw the structure of the enolate ion that is generated during the course of this reaction.
Refer to instructions. Draw the structure of the enolate ion that is generated during the course of this reaction.
(Essay)
4.8/5  (34)
(34)
When treated with base and heat, the following diketone undergoes an intramolecular aldol reaction followed by dehydration to produce cis-jasmone, a perfume component. Draw the structure of the product.

(Essay)
4.9/5  (38)
(38)
Instructions: Draw the structure of the product you would expect to obtain by Claisen condensation of the esters shown in the question(s) below. If an ester does not undergo Claisen condensation, explain why it does not.
Draw and explain:
(Essay)
4.7/5  (45)
(45)
Instructions: Consider the structures below to answer the following question(s).  Refer to instructions. Underline the acidic hydrogen atoms in each of the molecules.
Refer to instructions. Underline the acidic hydrogen atoms in each of the molecules.
(Essay)
5.0/5  (30)
(30)
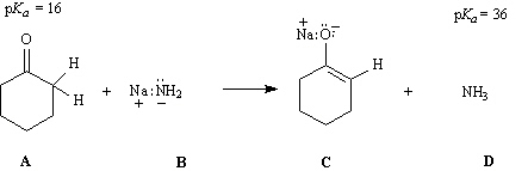
Consider the reaction below to answer the following questions.
 Refer to Instructions. The weakest acid in the reaction is:
Refer to Instructions. The weakest acid in the reaction is:
(Short Answer)
4.9/5  (30)
(30)
Instructions: Consider the reaction below to answer the following question(s).
 Refer to instructions. This reaction is an example of:
Refer to instructions. This reaction is an example of:
(Multiple Choice)
4.7/5  (37)
(37)
Which of the following would form an enolate ion on treatment with a base? 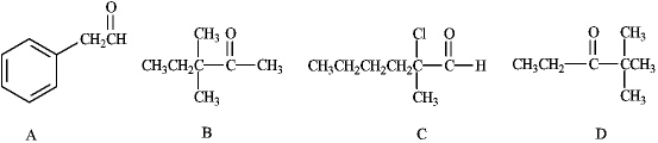
(Multiple Choice)
4.8/5  (46)
(46)
Instructions: Refer to the compounds below to answer the following question(s).  Refer to instructions. Underline all the acidic hydrogen atoms in Compounds I through IV.
Refer to instructions. Underline all the acidic hydrogen atoms in Compounds I through IV.
(Essay)
4.8/5  (42)
(42)
Showing 1 - 20 of 37
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)