Exam 13: Structure Determination
Exam 1: Structure and Bonding:acids and Bases40 Questions
Exam 2: Alkanes: The Nature of Organic Compounds47 Questions
Exam 3: Alkenes and Alkynes: the Nature of Organic Reactions37 Questions
Exam 4: Reactions of Alkenes and Alkynes44 Questions
Exam 5: Aromatic Compounds51 Questions
Exam 6: Sterechemistry at Tetrahedral Centers35 Questions
Exam 7: Organohalides: Nucleophilic Substitutions and Eliminations49 Questions
Exam 8: Alcohols, Phenols, Ethers, and Their Sulfur Analogs39 Questions
Exam 9: Aldehydes and Ketones: Nucleophilic Addition Reactions32 Questions
Exam 10: Carboxylic Acids and Derivatives: Nucleophilic Acyl Substitution Reactions66 Questions
Exam 11: Carbonyl Alpha-Substitution Reactions and Condensation Reactions37 Questions
Exam 12: Amines31 Questions
Exam 13: Structure Determination64 Questions
Exam 14: Biomolecules: Carbohydrates41 Questions
Exam 15: Biomolecules: Amino Acids, Peptides, and Proteins43 Questions
Exam 16: Biomolecules: Lipids and Nucleic Acids37 Questions
Exam 17: The Organic Chemistry of Metabolic Pathways35 Questions
Select questions type
Which feature in the 1H NMR spectrum provides information about the electronic environment of the protons in a compound?
Free
(Multiple Choice)
4.9/5  (33)
(33)
Correct Answer:
D
Which of the following compounds gives a 1H NMR spectrum consisting of only two singlets?
Free
(Multiple Choice)
4.8/5  (37)
(37)
Correct Answer:
C
Which of the following bonds undergoes stretching at the highest frequency?
Free
(Multiple Choice)
4.8/5  (36)
(36)
Correct Answer:
A
Instructions: Answer the following question(s) for the compound whose 1H NMR spectra is shown below.
C4H8O 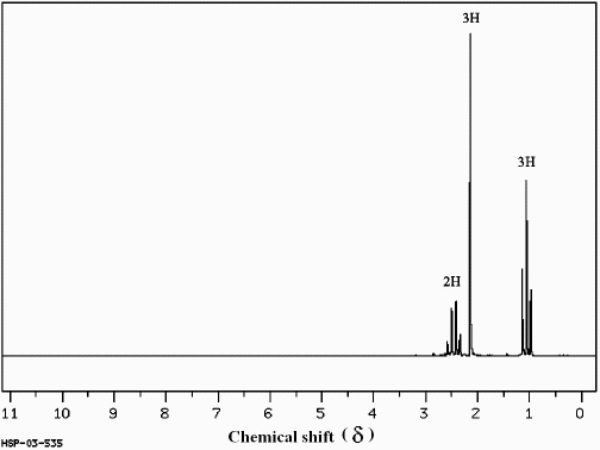 (Spectrum obtained from: SDBSWeb: http://www.aist.go.jp/RIODB/SDBS/)
Refer to instructions. Describe each signal in terms of its integration, splitting and chemical shift.
(Spectrum obtained from: SDBSWeb: http://www.aist.go.jp/RIODB/SDBS/)
Refer to instructions. Describe each signal in terms of its integration, splitting and chemical shift.
(Essay)
4.7/5  (36)
(36)
Assume you are carrying out the conversion of 1-bromobutane to butan-1-ol. How could you use IR spectroscopy to determine when the reaction is complete? 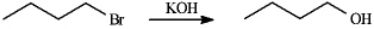
(Essay)
4.9/5  (41)
(41)
Instructions: The following questions pertain to the charting of NMR spectra. MATCH a term to each description below. Place the letter of the term in the blank to the left of the description.
-Refer to instructions. __________ The NMR charts are calibrated using an arbitrary scale that is divided into __________ units.
(Multiple Choice)
4.9/5  (35)
(35)
Which of the following regions in the electromagnetic spectrum corresponds to the radiation with the highest energy?
(Multiple Choice)
5.0/5  (42)
(42)
Which feature in the 1H NMR spectrum provides information about the relative numbers of hydrogen atoms of each type found in a compound?
(Multiple Choice)
4.9/5  (36)
(36)
Examining the infrared spectrum of a compound allows us to:
(Multiple Choice)
4.7/5  (37)
(37)
When an organic molecule is irradiated with ultraviolet radiation, the energy absorbed by the molecule corresponds to:
a. the amount necessary to increase molecular motions in functional groups
b. the amount necessary to excite electrons from one molecular orbital to another
c. the amount necessary to "flip" the spin of atomic nuclei
d. the amount necessary to strip a molecule of one electron to generate a radical cation
(Short Answer)
4.8/5  (39)
(39)
Which of the following would produce only singlets in an 1H NMR spectrum? 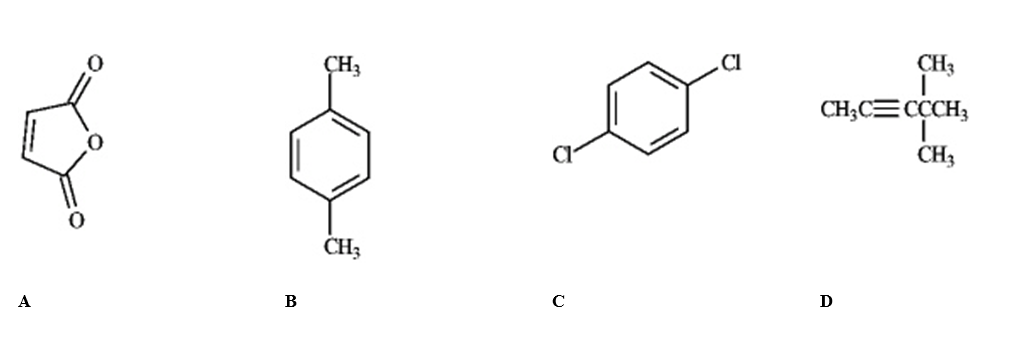
(Multiple Choice)
4.8/5  (43)
(43)
Which of the following compounds gives an infrared spectrum with peaks at 3300 cm-1 (sharp peak) and 2150 cm-1 (sharp peak)?
(Multiple Choice)
4.7/5  (50)
(50)
Instructions: Refer to the mass spectrum of 2-methylbutane shown below to answer the following question(s). 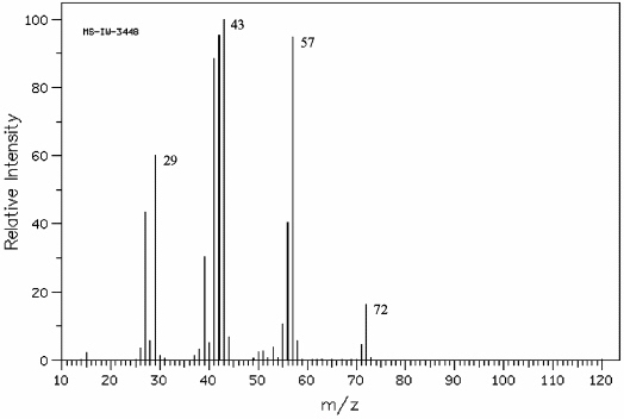 (Spectrum obtained from: SDBSWeb: http://www.aist.go.jp/RIODB/SDBS/)
Refer to instructions. What peak represents the base peak?
(Spectrum obtained from: SDBSWeb: http://www.aist.go.jp/RIODB/SDBS/)
Refer to instructions. What peak represents the base peak?
(Essay)
4.9/5  (45)
(45)
Instructions: For each of the compounds below tell how many signals you would expect the molecule to have in its normal, broadband decoupled 13C NMR spectra.
Number of signals: 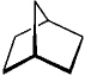
(Essay)
4.8/5  (40)
(40)
Instructions: Refer to the mass spectrum of 2-methylbutane shown below to answer the following question(s). 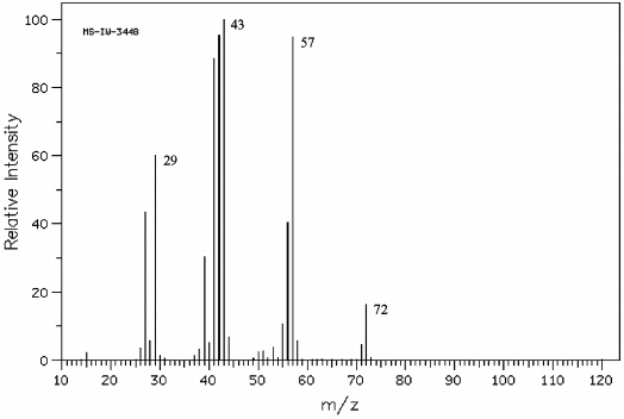 (Spectrum obtained from: SDBSWeb: http://www.aist.go.jp/RIODB/SDBS/)
Refer to instructions. What peak represents M+?
(Spectrum obtained from: SDBSWeb: http://www.aist.go.jp/RIODB/SDBS/)
Refer to instructions. What peak represents M+?
(Essay)
4.8/5  (40)
(40)
Instructions: Predict the splitting patterns you would expect for each proton in the molecules below:
Predict: 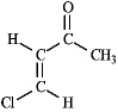
(Essay)
4.8/5  (37)
(37)
Instructions: Match each of the following groups of bond-types to the region of the infrared spectrum in which their absorptions occur.

(Essay)
4.9/5  (36)
(36)
Instructions: The following questions pertain to the charting of NMR spectra. MATCH a term to each description below. Place the letter of the term in the blank to the left of the description.
-Refer to instructions. __________ When looking at an NMR chart the right-hand part of the chart is the __________.
(Multiple Choice)
4.8/5  (45)
(45)
Which of the following compounds gives an infrared spectrum with peaks at 3300 cm-1 (strong, broad peak) and 1640 cm-1 (sharp, weak peak)?
(Multiple Choice)
4.8/5  (36)
(36)
Showing 1 - 20 of 64
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)