Exam 2: Atoms, Ions, and Compounds
Exam 1: Matter, Energy, and the Origins of the Universe77 Questions
Exam 2: Atoms, Ions, and Compounds102 Questions
Exam 3: Chemical Reactions and Earths Composition97 Questions
Exam 4: Solution Chemistry and the Hydrosphere98 Questions
Exam 5: Thermochemistry101 Questions
Exam 6: Properties of Gases: the Air We Breathe106 Questions
Exam 7: Electrons in Atoms and Periodic Properties104 Questions
Exam 8: Chemical Bonding and Climate Change104 Questions
Exam 9: Molecular Geometry and Bonding Theories101 Questions
Exam 10: Forces Between Ions and Molecules100 Questions
Exam 11: Solutions and Their Colligative Properties92 Questions
Exam 12: The Chemistry of Solids128 Questions
Exam 13: Organic Chemistry: Fuels, Pharmaceuticals, and Materials112 Questions
Exam 14: Thermodynamics: Spontaneous Processes, Entropy, and Free Energy79 Questions
Exam 15: Chemical Kinetics128 Questions
Exam 16: Chemical Equilibrium105 Questions
Exam 17: Equilibrium in the Aqueous Phase156 Questions
Exam 18: The Colorful Chemistry of Metals114 Questions
Exam 19: Electrochemistry and the Quest for Clean Energy103 Questions
Exam 20: Biochemistry: the Compounds of Life109 Questions
Exam 21: Nuclear Chemistry108 Questions
Exam 22: Life and the Periodic Table95 Questions
Select questions type
The average atomic mass of zinc is 65.39 amu. Given the data in the following table, what is the natural abundance of 66Zn? Isotope
Mass (amu)
Natural Abundance (%)64Zn
63)9291
48)8966Zn
65)9260
?67Zn
66)9271
4)1168Zn
67)9249
18)5670Zn
69)9253
0)62
Free
(Multiple Choice)
4.7/5  (44)
(44)
Correct Answer:
A
The average atomic mass of carbon is 12.01 amu. What is the average atomic mass of carbon in grams? (1 amu = 1.6605402 10-27 kg)
Free
(Essay)
4.9/5  (26)
(26)
Correct Answer:
1.994 10-23 g
When cosmic rays strike atoms in the upper atmosphere, energetic neutrons are produced. These neutrons collide with nitrogen-14 atoms, producing carbon-14 atoms and hydrogen atoms. Which diagram represents the carbon-14 product? 
Free
(Multiple Choice)
4.9/5  (27)
(27)
Correct Answer:
D
In the atoms in the Rutherford-Geiger-Marsden experiment, the particles were repelled by __________
(Multiple Choice)
4.8/5  (43)
(43)
Which contains more carbon by mass, 1 g of CO2 or 1 g of CO?
(Multiple Choice)
4.9/5  (39)
(39)
A hypothetical element has two stable isotopes: one isotope has a mass of 106.9051 amu with an abundance of 48.183%, the other isotope has a mass of 108.9048 amu with an abundance of 51.825%. What is the average atomic mass of this element?
(Multiple Choice)
4.9/5  (40)
(40)
Nuclear reactors used for power generation require uranium enriched in uranium-235. What is the average atomic mass of enriched uranium consisting of exactly 3.0% uranium-235 (235.04 amu) and 97.0% uranium-238 (238.05 amu)?
(Short Answer)
4.7/5  (40)
(40)
Nitrogen and oxygen combine to form several different nitrogen oxides. In one case, 8.4 g of nitrogen reacted completely with 4.8 g of oxygen. In another case, 4.2 g of nitrogen reacted with 9.6 g of oxygen. Which pair of nitrogen oxides is consistent with these data?
(Multiple Choice)
4.9/5  (36)
(36)
Rutherford, Geiger, and Marsden's experiment demonstrated that the volume of the nucleus is roughly what fraction of the volume occupied by the electrons?
(Multiple Choice)
4.8/5  (39)
(39)
Manganese(IV) oxide is a brown insoluble solid often found as a product of reactions of potassium permanganate. What is the formula of manganese(IV) oxide?
(Multiple Choice)
4.9/5  (39)
(39)
A 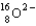 ion has __________ protons, __________ neutrons, and __________ electrons.
ion has __________ protons, __________ neutrons, and __________ electrons.
(Multiple Choice)
4.8/5  (31)
(31)
Showing 1 - 20 of 102
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)