Exam 18: Solubility and Complex-Ion Equilibria
Exam 1: Matter - Its Properties and Measurement94 Questions
Exam 2: Atoms and the Atomic Theory100 Questions
Exam 3: Chemical Compounds100 Questions
Exam 4: Chemical Reactions100 Questions
Exam 5: Introduction to Reactions in Aqueous Solutions97 Questions
Exam 6: Gases100 Questions
Exam 7: Thermochemistry101 Questions
Exam 8: Electrons in Atoms100 Questions
Exam 9: The Periodic Table and Some Atomic Properties96 Questions
Exam 10: Chemical Bonding I: Basic Concepts97 Questions
Exam 11: Chemical Bonding II: Additional Aspects97 Questions
Exam 12: Intermolecular Forces: Liquids and Solids102 Questions
Exam 13: Solutions and Their Physical Properties100 Questions
Exam 14: Chemical Kinetics92 Questions
Exam 15: Principles of Chemical Equilibrium99 Questions
Exam 16: Acids and Bases100 Questions
Exam 17: Additional Aspects of Acid-Base Equilibria99 Questions
Exam 18: Solubility and Complex-Ion Equilibria95 Questions
Exam 19: Spontaneous Change: Entropy and Free Energy101 Questions
Exam 20: Electrochemistry103 Questions
Exam 21: Main Group Elements I: Groups 1, 2, 13, and 14116 Questions
Exam 22: Main Group Elements II: Groups 18, 17, 16, 15, and Hydrogen100 Questions
Exam 23: The Transition Elements102 Questions
Exam 24: Complex Ions and Coordination Compounds100 Questions
Exam 25: Nuclear Chemistry 1-41100 Questions
Exam 26: Structures of Organic Compounds96 Questions
Exam 27: Reactions of Organic Compounds94 Questions
Exam 28: Chemistry of the Living State99 Questions
Select questions type
Which of the following soil additives would best reduce the concentration of dissolved Fe3+ in ground water?
(Multiple Choice)
4.8/5  (41)
(41)
A homeowner in Boston becomes concerned that there may be appreciable amounts of lead in her drinking water due to 150-year old water pipes in her house. Consequently, she takes a sample of her drinking water in to be analyzed. The laboratory technician, who is new on the job, has been told to analyze by precipitating the lead ion as the iodide (Ksp = 7.1 × 10-9 for PbI2) by slowly adding small portions of 1.00 M NaI solution. If we assume that the concentration of lead in the solution is 1.00 mg/liter or approximately 4.8 × 10-6 M (this would be 1.0 part per million) is it possible to detect the lead in the drinking water by adding a total of no more than 10.0 mL of NaI solution to a 100 mL sample of drinking water? You must of course consider dilution effects. What is Q?
(Multiple Choice)
4.9/5  (40)
(40)
When solid silver chloride is shaken with a 0.1 molar solution of potassium iodide, most of the silver chloride is converted to silver iodide. This transformation takes place because:
(Multiple Choice)
4.7/5  (42)
(42)
A solution contains [Ba2+] = 5.0 × 10-5 M, [Ag+] = 3.0 × 10-5 M, and [Zn2+] = 2.0 × 10-7 M. Sodium oxalate is slowly added so that [C2O42-] increases.
Salt BaC2O4 ZnC2O4 Ag2C2O4
Ksp 1.5 × 10-8 1.35 × 10-9 1.1 × 10-11
Which one of the oxalates precipitates first?
(Multiple Choice)
4.8/5  (34)
(34)
If iron(III) acetate is added to be 1 × 10-10 M in a solution that is 0.10 M NH3, what is Qsp and will Fe(OH)3 precipitate? The Ksp of Fe(OH)3 is 4 × 10-38 and Kb for NH3 is 1.8 × 10-5.
(Multiple Choice)
4.7/5  (32)
(32)
Predict the molar solubility of the following salt in a solution that contains the given concentration of one of its ions: BaSO4; [SO42-] = 4.3 × 10-5 M; Ksp = 1.1 × 10-10
(Multiple Choice)
4.8/5  (41)
(41)
The solubility product constant of Mg(OH)2 is 9.0 × 10-12. If a solution is 0.010 M with respect to Mg2+ ion, the amount of [OH-] required to start the precipitation of Mg(OH)2 is:
(Multiple Choice)
4.8/5  (42)
(42)
In which of the following solutions will the concentration of dissolved silver species be the highest if solid AgNO3 is stirred with 1.00 L of solution?
(Multiple Choice)
4.8/5  (32)
(32)
The solubility of cerium iodate, Ce(IO3)3, molar mass = 664.83 g/mol, in pure water is 124 mg per 100 mL of water. Calculate the solubility product constant for cerium iodate.
(Multiple Choice)
4.7/5  (40)
(40)
Some solid in a solution at equilibrium means the solution is saturated.
(True/False)
4.8/5  (33)
(33)
What is the composition of the precipitate formed when H2S gas is bubbled through 1.0 litre of a solution of 0.010 M Zn2+, 0.010 M Pb2+, and 0.010 M Mn2+, buffered at pH 2.0, until 0.10 mol of H2S has been added? [Ksp values are: ZnS: 1.6 × 10-23; MnS: 7.0 × 10-16; PbS: 7.0 × 10-29. For H2S, Ka1 = 1.0 × 10-7; Ka2 = 1.3 × 10-13.
(Multiple Choice)
4.8/5  (45)
(45)
Which of the following salts, each of which has a solubility product equal to 1.0 × 10-6, has the greatest molar solubility?
(Multiple Choice)
4.8/5  (37)
(37)
Write the solubility product constant expression for the following salt: PbC2O4.
(Multiple Choice)
4.9/5  (43)
(43)
When 200 mL of 0.10 M BaCl2 is added to 100 mL of 0.30 M Na2SO4, the number of moles of BaSO4 (solubility product = 1.1 × 10-10) precipitated is ________.
(Multiple Choice)
4.8/5  (38)
(38)
When equal volumes of the indicated solutions are mixed, precipitation should occur only for: 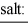
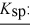 barium fluoride 1.0 × 10-6 calcium carbonate 2.8 × 10-9
Calcium fluoride 5.3 × 10-9
Magnesium fluoride 3.7 × 10-8
Silver carbonate 8.5 × 10-12
barium fluoride 1.0 × 10-6 calcium carbonate 2.8 × 10-9
Calcium fluoride 5.3 × 10-9
Magnesium fluoride 3.7 × 10-8
Silver carbonate 8.5 × 10-12
(Multiple Choice)
4.8/5  (34)
(34)
When equal volumes of the indicated solutions are mixed, precipitation should occur only for: 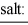
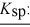 barium fluoride 1.0 × 10-6 calcium carbonate 2.8 × 10-9
Calcium fluoride 5.3 × 10-9
Magnesium fluoride 3.7 × 10-9
Silver carbonate 8.5 × 10-12
barium fluoride 1.0 × 10-6 calcium carbonate 2.8 × 10-9
Calcium fluoride 5.3 × 10-9
Magnesium fluoride 3.7 × 10-9
Silver carbonate 8.5 × 10-12
(Multiple Choice)
4.7/5  (30)
(30)
What ratio of [NH4+]/[NH3] would provide a buffer of pH low enough to avoid precipitation of Co(OH)2 [Ksp = 2.0 × 10-14] from a 0.50 M Co2+ solution? [Kb for NH3 = 1.8 × 10-5]
(Multiple Choice)
4.8/5  (35)
(35)
What is the concentration of Ca2+ in ppm (mg/L) in cave water saturated with calcite (calcium carbonate, Ksp = 4.7 × 10-9)?
(Multiple Choice)
4.9/5  (43)
(43)
What is the free Cu2+ concentration if 0.020 M Cu2+ solution is mixed with an equal volume of 4.0 M NH3? Kf for [Cu(NH3)4]2+ is 1.1 × 1013.
(Multiple Choice)
4.9/5  (32)
(32)
Showing 41 - 60 of 95
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)