Exam 12: Intermolecular Forces: Liquids and Solids
Exam 1: Matter - Its Properties and Measurement94 Questions
Exam 2: Atoms and the Atomic Theory100 Questions
Exam 3: Chemical Compounds100 Questions
Exam 4: Chemical Reactions100 Questions
Exam 5: Introduction to Reactions in Aqueous Solutions97 Questions
Exam 6: Gases100 Questions
Exam 7: Thermochemistry101 Questions
Exam 8: Electrons in Atoms100 Questions
Exam 9: The Periodic Table and Some Atomic Properties96 Questions
Exam 10: Chemical Bonding I: Basic Concepts97 Questions
Exam 11: Chemical Bonding II: Additional Aspects97 Questions
Exam 12: Intermolecular Forces: Liquids and Solids102 Questions
Exam 13: Solutions and Their Physical Properties100 Questions
Exam 14: Chemical Kinetics92 Questions
Exam 15: Principles of Chemical Equilibrium99 Questions
Exam 16: Acids and Bases100 Questions
Exam 17: Additional Aspects of Acid-Base Equilibria99 Questions
Exam 18: Solubility and Complex-Ion Equilibria95 Questions
Exam 19: Spontaneous Change: Entropy and Free Energy101 Questions
Exam 20: Electrochemistry103 Questions
Exam 21: Main Group Elements I: Groups 1, 2, 13, and 14116 Questions
Exam 22: Main Group Elements II: Groups 18, 17, 16, 15, and Hydrogen100 Questions
Exam 23: The Transition Elements102 Questions
Exam 24: Complex Ions and Coordination Compounds100 Questions
Exam 25: Nuclear Chemistry 1-41100 Questions
Exam 26: Structures of Organic Compounds96 Questions
Exam 27: Reactions of Organic Compounds94 Questions
Exam 28: Chemistry of the Living State99 Questions
Select questions type
The factor that has the largest effect on vapor pressure is ________.
(Multiple Choice)
4.8/5  (33)
(33)
The maximum temperature at which a gas can be liquefied is just below the ________.
(Multiple Choice)
4.9/5  (43)
(43)
Below are given the Lewis structures of five molecules. Which one displays the MOST hydrogen bonding?
(Multiple Choice)
4.9/5  (41)
(41)
There are no types of crystalline solids that are held together by:
(Multiple Choice)
4.7/5  (38)
(38)
According to the phase diagram given, which of the following statements is wrong? 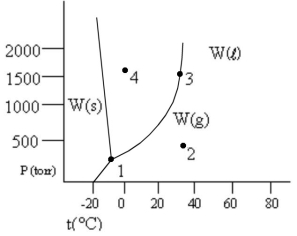
(Multiple Choice)
4.8/5  (38)
(38)
Which of the following compounds has the largest lattice energy? MgCO3, Na2CO3, Al2(CO3)2
(Multiple Choice)
4.9/5  (43)
(43)
What is the difference between "normal boiling point" and "boiling point" of a liquid?
(Multiple Choice)
4.9/5  (45)
(45)
A crystal does not conduct electricity, even after melting. It is hard and brittle and melts at a very high temperature. What type of crystal is it?
(Multiple Choice)
4.8/5  (31)
(31)
A crystal does not conduct electricity, yet its melt and aqueous solutions do. It is hard and brittle and melts at a high temperature. What type of crystal is it?
(Multiple Choice)
4.8/5  (42)
(42)
A 0.90 g sample of liquid water was introduced into an evacuated 2.00 L flask, which was then sealed and heated to 37°C. What percentage, by mass, of the water remained as liquid? [Vapor pressure of water at 37°C = 48.2 torr.]
(Multiple Choice)
4.9/5  (34)
(34)
The normal boiling point of acetone is 56.2°C and the molar heat of vaporization is 32.0 kJ/mol. At what temperature will acetone boil under a pressure of 50.0 mmHg?
(Multiple Choice)
4.8/5  (34)
(34)
All sides are equal in length and all angles are right angles. There is an atom at each corner and one at the center of each side. This is a ________.
(Multiple Choice)
4.9/5  (42)
(42)
A substance has a heat of fusion of 61.5 kJ/mol and a heat of deposition of -167.4 kJ/mol. What is the heat of sublimation in kJ/mol?
(Multiple Choice)
4.8/5  (44)
(44)
A crystal and its melt readily conduct electricity. The crystal also has a luster and is easily deformed. Thus, it is:
(Multiple Choice)
4.8/5  (36)
(36)
The enthalpy of vaporization at 298 K for diethylether (C4H10O) is 26.0 kJ/mol. How much heat would be required to vaporize 1.00 L of the ether at 298 K if its density is 0.714 g/L?
(Multiple Choice)
4.8/5  (44)
(44)
Which probably has the highest boiling point at 1.00 atm pressure?
(Multiple Choice)
4.9/5  (38)
(38)
Which of the following statements concerning molecules in the liquid state is true?
(Multiple Choice)
4.8/5  (40)
(40)
Which of the following is true assuming point A has P = 180 mmHg, T = 50°C, point C has P = 275 mmHg, T = 200°C and points A, B, and C form a right triangle? 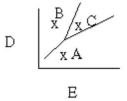
(Multiple Choice)
4.8/5  (40)
(40)
Showing 61 - 80 of 102
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)