Exam 12: Intermolecular Forces: Liquids and Solids
Exam 1: Matter - Its Properties and Measurement94 Questions
Exam 2: Atoms and the Atomic Theory100 Questions
Exam 3: Chemical Compounds100 Questions
Exam 4: Chemical Reactions100 Questions
Exam 5: Introduction to Reactions in Aqueous Solutions97 Questions
Exam 6: Gases100 Questions
Exam 7: Thermochemistry101 Questions
Exam 8: Electrons in Atoms100 Questions
Exam 9: The Periodic Table and Some Atomic Properties96 Questions
Exam 10: Chemical Bonding I: Basic Concepts97 Questions
Exam 11: Chemical Bonding II: Additional Aspects97 Questions
Exam 12: Intermolecular Forces: Liquids and Solids102 Questions
Exam 13: Solutions and Their Physical Properties100 Questions
Exam 14: Chemical Kinetics92 Questions
Exam 15: Principles of Chemical Equilibrium99 Questions
Exam 16: Acids and Bases100 Questions
Exam 17: Additional Aspects of Acid-Base Equilibria99 Questions
Exam 18: Solubility and Complex-Ion Equilibria95 Questions
Exam 19: Spontaneous Change: Entropy and Free Energy101 Questions
Exam 20: Electrochemistry103 Questions
Exam 21: Main Group Elements I: Groups 1, 2, 13, and 14116 Questions
Exam 22: Main Group Elements II: Groups 18, 17, 16, 15, and Hydrogen100 Questions
Exam 23: The Transition Elements102 Questions
Exam 24: Complex Ions and Coordination Compounds100 Questions
Exam 25: Nuclear Chemistry 1-41100 Questions
Exam 26: Structures of Organic Compounds96 Questions
Exam 27: Reactions of Organic Compounds94 Questions
Exam 28: Chemistry of the Living State99 Questions
Select questions type
Which of the following statements about viscosity are true: I) Viscosity is liquid's resistance to flow.
II) Viscosity decreases with a decrease in temperature.
III) Viscosity is not related to the forces between molecules in a liquid.
IV) Viscous liquids have low rate flows.
(Multiple Choice)
4.9/5  (39)
(39)
If 34 g of a solid of mol mass = 174 g/mol requires 21.3 kJ to melt it, what is the molar heat of fusion in kJ/mol?
(Multiple Choice)
5.0/5  (34)
(34)
A liquid has a normal boiling point of 78°C and its vapor pressure is 400 mmHg at 50°C. To compute the molar heat of vaporization, one needs:
(Multiple Choice)
4.7/5  (44)
(44)
Given the following information, calculate ΔH°(in kcal mol-1) for: I(g) + e- → I-(g)
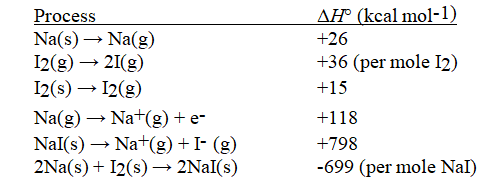
(Multiple Choice)
4.9/5  (39)
(39)
Given the following information, calculate ΔH°(in kcal mol-1) for:
I(g) + e- → I-(g) 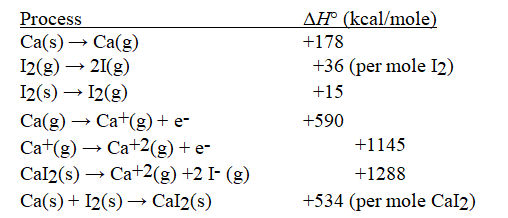
(Multiple Choice)
4.8/5  (30)
(30)
All sides are equal in length and all angles are right angles. There is an atom at each corner and one in the center. This is a simple cubic cell.
(True/False)
4.9/5  (41)
(41)
A liquid has a molar heat of vaporization of 22.7 kJ/mol. Its normal boiling point is 459K. What is the vapor pressure, in mmHg, at 70°C?
(Multiple Choice)
4.8/5  (28)
(28)
According to the phase diagram given, which of the following is INCORRECT? 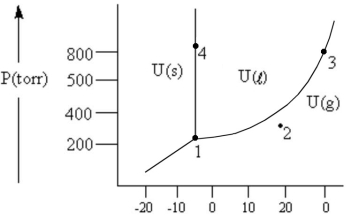
(Multiple Choice)
4.7/5  (40)
(40)
The enthalpy of condensation is equal to, but opposite in sign from, the enthalpy of ________.
(Multiple Choice)
4.8/5  (47)
(47)
Arrange in order by decreasing boiling point: CH3CH2OH, HOCH2CH2OH, C4H10
(Multiple Choice)
4.8/5  (44)
(44)
If one compares compound A, composed of nonpolar molecules, with compound B, composed of polar molecules, and both molecules have the same molecular formula, then it is true that:
(Multiple Choice)
4.8/5  (30)
(30)
A liquid is in equilibrium with its vapor. If some of the vapor is allowed to escape, what is the immediate result?
(Multiple Choice)
4.9/5  (28)
(28)
Given the tabulated data, what is the electron affinity for chlorine? heat of sublimation for calcium +178 kJ/mol
First ionization energy for calcium +590 kJ/mol
Second ionization energy for calcium +1145 kJ/mol
Heat of dissociation for chlorine +122 kJ/mol(Cl)
Heat of formation for calcium chloride -796 kJ/mol
Lattice energy of crystalline calcium chloride -2255 kJ/mol
(Multiple Choice)
4.9/5  (48)
(48)
Arrange the following compounds in order of increasing boiling point:
I) 1-propanol, CH3CH2CH2OH;
II) 1,2-propanediol, CH3CH(OH)CH2OH;
III) 1,2,3-propanetriol, (glycerol), HOCH2CH(OH)CH2OH.
(Multiple Choice)
4.9/5  (40)
(40)
List the following ionic compounds in order of increasing solubility in water: RbI, CaO, KCl
(Multiple Choice)
4.9/5  (38)
(38)
Given the following information, calculate ΔH°(in kcal mol-1) for: CaI2(s) → Ca+2(g) +2 I-(g)
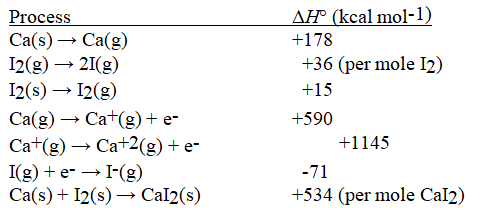
(Multiple Choice)
4.8/5  (36)
(36)
Showing 21 - 40 of 102
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)