Exam 15: Aldehydes and Ketones
Exam 1: Basic Concepts About Matter70 Questions
Exam 2: Measurements in Chemistry44 Questions
Exam 3: Atomic Structure and the Periodic Table70 Questions
Exam 4: Chemical Bonding: the Ionic Bond Model70 Questions
Exam 5: Chemical Bonding: the Covalent Bond Model70 Questions
Exam 6: Chemical Calculations: Formula Masses, Moles, and Chemical Equations70 Questions
Exam 7: Gases, Liquids, and Solids65 Questions
Exam 8: Solutions69 Questions
Exam 9: Chemical Reactions66 Questions
Exam 10: Acids, Bases, and Salts70 Questions
Exam 11: Nuclear Chemistry70 Questions
Exam 12: Saturated Hydrocarbons70 Questions
Exam 13: Unsaturated Hydrocarbons70 Questions
Exam 14: Alcohols, Phenols, and Ethers70 Questions
Exam 15: Aldehydes and Ketones66 Questions
Exam 16: Carboxylic Acids, Esters, and Other Acid Derivatives64 Questions
Exam 17: Amines and Amides54 Questions
Exam 18: Carbohydrates70 Questions
Exam 19: Lipids70 Questions
Exam 20: Proteins65 Questions
Exam 21: Enzymes and Vitamins70 Questions
Exam 22: Nucleic Acids64 Questions
Exam 23: Biochemical Energy Production70 Questions
Exam 24: Carbohydrate Metabolism70 Questions
Exam 25: Lipid Metabolism65 Questions
Exam 26: Protein Metabolism70 Questions
Select questions type
Use the following to answer the questions below:
For each of the compound characterizations, select from the response list the number of constitutional isomers that exist. Responses may be used more than once or need not be used at all.
-Molecular formula of C4H8O
Free
(Multiple Choice)
4.7/5  (39)
(39)
Correct Answer:
B
What two functional groups are never found at the end of a carbon chain?
Free
(Multiple Choice)
4.9/5  (28)
(28)
Correct Answer:
D
Which of the following structural features is common to both aldehydes and ketones?
Free
(Multiple Choice)
4.9/5  (39)
(39)
Correct Answer:
C
Which of the following statements concerning a carbonyl group is incorrect?
(Multiple Choice)
4.8/5  (27)
(27)
Use the following to answer the questions below:
In each of the following multiple-choice questions, characterize EACH of the three given statements as being TRUE or FALSE and then indicate the collective true-false status of the statements using the choices:
-Statements: (1) Butanal and 2-butanone are constitutional isomers.
(2) The linear notation for an aldehyde is RCOH and that for a ketone is RCOR.
(3) Water molecules can hydrogen bond with aldehyde and ketone molecules.
(Multiple Choice)
4.8/5  (37)
(37)
Which of the following compounds would be resistant to oxidation with a mild oxidizing agent?
(Multiple Choice)
4.9/5  (46)
(46)
Use the following to answer the questions below:
For each pair of compounds, select a correct characterization from the response list. Responses may be used more than once or need not be used at all.
-Diethylketone Pentanal
(Multiple Choice)
4.8/5  (24)
(24)
Use the following to answer the questions below:
For each set of reactants, select a correct product characterization from the response list. Responses may be used more than once or need not be used at all.
-Hemiacetal, alcohol, acid catalyst
(Multiple Choice)
4.8/5  (34)
(34)
Use the following to answer the questions below:
In each of the following multiple-choice questions, characterize EACH of the three given statements as being TRUE or FALSE and then indicate the collective true-false status of the statements using the choices:
-Statements: (1) Cyclic hemiacetals are usually more stable than noncyclic hemiacetals.
(2) A carbonyl group consists of a carbon atom and an oxygen atom joined by a double bond.
(3) The compound benzaldehyde contain six carbon atoms.
(Multiple Choice)
4.9/5  (33)
(33)
Use the following to answer the questions below:
For each of the reaction types, select from the response list the type of organic compound produced. Responses may be used more than once or need not be used at all.
-Oxidation of 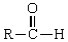
(Multiple Choice)
4.9/5  (43)
(43)
Use the following to answer the questions below:
For each of the reaction types, select from the response list the type of organic compound produced. Responses may be used more than once or need not be used at all.
-Oxidation of 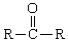
(Multiple Choice)
4.9/5  (40)
(40)
When an alcohol molecule adds across a carbon-oxygen double bond of an aldehyde or ketone, the
(Multiple Choice)
4.8/5  (47)
(47)
Which of the following statements concerning aldehydes and ketones is correct?
(Multiple Choice)
4.8/5  (37)
(37)
Use the following to answer the questions below:
In each of the following multiple-choice questions, characterize EACH of the three given statements as being TRUE or FALSE and then indicate the collective true-false status of the statements using the choices:
-Statements: (1) The compound 4-oxopentanal contains both an aldehyde and an ether functional group.
(2) An alkoxy group and a hydroxy group attached to the same carbon atom are present in both hemiacetals and acetals.
(3) Cyclic aldehyde structures are possible but cyclic ketone structures are not possible.
(Multiple Choice)
4.8/5  (33)
(33)
Which of the following compounds is a constitutional isomer of 2-pentanone?
(Multiple Choice)
4.8/5  (30)
(30)
The simplest aldehyde and ketone each contain how many carbon atoms, respectively?
(Multiple Choice)
4.7/5  (22)
(22)
Which of the following pairings of aldehyde common and IUPAC names is correct?
(Multiple Choice)
4.9/5  (28)
(28)
Which of the following structural features is possessed by aldehydes but not ketones?
(Multiple Choice)
4.8/5  (40)
(40)
Use the following to answer the questions below:
For each set of reactants, select a correct product characterization from the response list. Responses may be used more than once or need not be used at all.
-Ketone, two alcohols, acid catalyst
(Multiple Choice)
4.9/5  (31)
(31)
Showing 1 - 20 of 66
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)