Exam 9: Chemical Reactions
Exam 1: Basic Concepts About Matter70 Questions
Exam 2: Measurements in Chemistry44 Questions
Exam 3: Atomic Structure and the Periodic Table70 Questions
Exam 4: Chemical Bonding: the Ionic Bond Model70 Questions
Exam 5: Chemical Bonding: the Covalent Bond Model70 Questions
Exam 6: Chemical Calculations: Formula Masses, Moles, and Chemical Equations70 Questions
Exam 7: Gases, Liquids, and Solids65 Questions
Exam 8: Solutions69 Questions
Exam 9: Chemical Reactions66 Questions
Exam 10: Acids, Bases, and Salts70 Questions
Exam 11: Nuclear Chemistry70 Questions
Exam 12: Saturated Hydrocarbons70 Questions
Exam 13: Unsaturated Hydrocarbons70 Questions
Exam 14: Alcohols, Phenols, and Ethers70 Questions
Exam 15: Aldehydes and Ketones66 Questions
Exam 16: Carboxylic Acids, Esters, and Other Acid Derivatives64 Questions
Exam 17: Amines and Amides54 Questions
Exam 18: Carbohydrates70 Questions
Exam 19: Lipids70 Questions
Exam 20: Proteins65 Questions
Exam 21: Enzymes and Vitamins70 Questions
Exam 22: Nucleic Acids64 Questions
Exam 23: Biochemical Energy Production70 Questions
Exam 24: Carbohydrate Metabolism70 Questions
Exam 25: Lipid Metabolism65 Questions
Exam 26: Protein Metabolism70 Questions
Select questions type
According to Le Chatelier's principle, which of the following effects will occur if NH3 is removed from an equilibrium mixture governed by the equation: 
Free
(Multiple Choice)
4.9/5  (32)
(32)
Correct Answer:
D
For the indicated element, select the correct oxidation number from the response list: oxidation number of Cr in Cr2O72-+.
Free
(Multiple Choice)
4.7/5  (33)
(33)
Correct Answer:
D
Consider the following equilibrim:  Determine the effect on the position of equilibrium when the temperature is decreased.
Determine the effect on the position of equilibrium when the temperature is decreased.
(Multiple Choice)
4.7/5  (48)
(48)
Assign the following reaction to one of the reaction classifications given in the response list: 4PH3 + Ni(CO)4 4CO + Ni(PH3)4
(Multiple Choice)
4.9/5  (41)
(41)
Consider the following redox equation: SO2 + NO2 SO3 + NO, choose an appropriate chemical formula from the list that identifies the substance oxidized.
(Multiple Choice)
4.8/5  (40)
(40)
Whether a reaction is exothermic or endothermic is determined by
(Multiple Choice)
4.8/5  (33)
(33)
Consider the following redox equation: SO2 + NO2 SO3 + NO, choose an appropriate chemical formula from the list that identifies the substance that loses electrons.
(Multiple Choice)
4.7/5  (33)
(33)
Catalysts are correctly characterized by each of the following statements except one. The exception is:
(Multiple Choice)
4.8/5  (22)
(22)
Increasing the temperature at which a chemical reaction occurs
(Multiple Choice)
5.0/5  (31)
(31)
For the following general reaction equation, choose the correct form of the equilibrium constant expression from the response list: 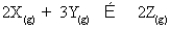
(Multiple Choice)
4.9/5  (36)
(36)
In which of the following sequences of sulfur-containing ions are the ions arranged in order of decreasing oxidation number for S?
(Multiple Choice)
4.9/5  (29)
(29)
Assign the following reaction to one of the reaction classifications given in the response list: K2CO3 K2O + CO2
(Multiple Choice)
4.8/5  (22)
(22)
Which substance functions as a reducing agent in the following redox reaction? CH4 + 2 O2 CO2 + 2H2O
(Multiple Choice)
4.8/5  (37)
(37)
Use the following to answer the questions below:
In each of the following multiple-choice questions, characterize EACH of the three given statements as being TRUE or FALSE and then indicate the collective true-false status of the statements using the choices:
-Characterize EACH of the three given statements as being TRUE or FALSE and then indicate the collective true-false status of the statements using the choices: (1) The square brackets associated with a general equilibrium constant expression mean that concentrations must be expressed in terms of molarity.
(2) Pressure changes affect all chemical systems at equilibrium in which gaseous reactants or products are present.
(3) In the compound KMnO4, the oxidation number of Mn is +3.
(Multiple Choice)
4.7/5  (39)
(39)
Which of the following statements concerning types of reactions is correct?
(Multiple Choice)
4.8/5  (28)
(28)
The proper assignment of oxidation numbers to the elements in the polyatomic ion SO32- would be
(Multiple Choice)
4.7/5  (35)
(35)
Showing 1 - 20 of 66
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)