Exam 13: Unsaturated Hydrocarbons
Exam 1: Basic Concepts About Matter70 Questions
Exam 2: Measurements in Chemistry44 Questions
Exam 3: Atomic Structure and the Periodic Table70 Questions
Exam 4: Chemical Bonding: the Ionic Bond Model70 Questions
Exam 5: Chemical Bonding: the Covalent Bond Model70 Questions
Exam 6: Chemical Calculations: Formula Masses, Moles, and Chemical Equations70 Questions
Exam 7: Gases, Liquids, and Solids65 Questions
Exam 8: Solutions69 Questions
Exam 9: Chemical Reactions66 Questions
Exam 10: Acids, Bases, and Salts70 Questions
Exam 11: Nuclear Chemistry70 Questions
Exam 12: Saturated Hydrocarbons70 Questions
Exam 13: Unsaturated Hydrocarbons70 Questions
Exam 14: Alcohols, Phenols, and Ethers70 Questions
Exam 15: Aldehydes and Ketones66 Questions
Exam 16: Carboxylic Acids, Esters, and Other Acid Derivatives64 Questions
Exam 17: Amines and Amides54 Questions
Exam 18: Carbohydrates70 Questions
Exam 19: Lipids70 Questions
Exam 20: Proteins65 Questions
Exam 21: Enzymes and Vitamins70 Questions
Exam 22: Nucleic Acids64 Questions
Exam 23: Biochemical Energy Production70 Questions
Exam 24: Carbohydrate Metabolism70 Questions
Exam 25: Lipid Metabolism65 Questions
Exam 26: Protein Metabolism70 Questions
Select questions type
For which of the following halogenated hydrocarbons is cis-trans isomerism possible?
Free
(Multiple Choice)
4.8/5  (35)
(35)
Correct Answer:
B
Characterize EACH of the three given statements as being TRUE or FALSE and then indicate the collective true-false status of the statements using the choices: (1) Ethene is both a plant hormone and a high-volume industrial chemical.
(2) Carotenoids, compounds which give odor to flowers and plants, have a system of conjugated double bonds as a structural feature.
(3) The process of vision in the human eye involves changes in which carbon-carbon double bonds are converted to carbon-carbon triple bonds.
Free
(Multiple Choice)
4.7/5  (34)
(34)
Correct Answer:
C
Which of the following addition polymers is produced from monomers that are dienes?
Free
(Multiple Choice)
4.9/5  (33)
(33)
Correct Answer:
D
Characterize EACH of the three given statements as being TRUE or FALSE and then indicate the collective true-false status of the statements using the choices: (1) Ethene and ethane molecules have the same geometrical shape.
(2) Two double bonds are present in the compound allyl chloride.
(3) Cis-trans isomerism is possible in both 1-butene and 2-butene.
(Multiple Choice)
4.8/5  (34)
(34)
A cycloalkene having only one carbon-carbon double bond will have the general formula
(Multiple Choice)
4.9/5  (35)
(35)
Which of the following reactions involving benzene require the presence of an AlCl3 catalyst?
(Multiple Choice)
5.0/5  (35)
(35)
Characterize EACH of the three given statements as being TRUE or FALSE and then indicate the collective true-false status of the statements using the choices: (1) Alkenes with two to four carbon atoms are gases at room temperature and pressure.
(2) Aromatic hydrocarbons do not readily undergo addition reactions.
(3) HDPE, LDPE, and Teflon are all ethene-based addition polymers.
(Multiple Choice)
4.8/5  (33)
(33)
For the following reaction situation, select a correct product characterization using the response list: Cyclohexene + one Cl2
(Multiple Choice)
4.7/5  (33)
(33)
For the pair of compounds, select a correct characterization from the response list: 1-butyne and 1,3-butadiene.
(Multiple Choice)
4.9/5  (33)
(33)
From the response list, select the correct molecular formula for 2-methylcyclohexene.
(Multiple Choice)
4.7/5  (39)
(39)
For the following polymer, select a correct polymer characterization using the response list: Teflon.
(Multiple Choice)
4.9/5  (33)
(33)
Which of the following is an incorrect IUPAC name for a disubstituted benzene?
(Multiple Choice)
4.8/5  (38)
(38)
For which of the following types of substituted benzenes is the specified number of constitutional isomers correct?
(Multiple Choice)
4.9/5  (36)
(36)
For the following reaction, select a correct product characterization from the response list. 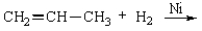
(Multiple Choice)
4.8/5  (34)
(34)
For the pair of compounds, select a correct characterization from the response list: 2-pentene and 2-butene.
(Multiple Choice)
4.9/5  (39)
(39)
For the following polymer, select a correct polymer characterization using the response list: natural rubber.
(Multiple Choice)
4.8/5  (31)
(31)
For the following reaction, select a correct product characterization from the response list. 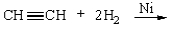
(Multiple Choice)
4.7/5  (33)
(33)
With the catalyst AlCl3 present, which reactant is needed to convert benzene to ethylbenzene?
(Multiple Choice)
4.8/5  (31)
(31)
Characterize EACH of the three given statements as being TRUE or FALSE and then indicate the collective true-false status of the statements using the choices: (1) o-xylene is a benzene derivative with two methyl substituents.
(2) A number is not needed to specify double bond position in the IUPAC names of ethene and propene.
(3) Hydrohalogenation is a symmetrical addition reaction.
(Multiple Choice)
4.9/5  (26)
(26)
Showing 1 - 20 of 70
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)