Exam 12: Quantum Mechanics and Atomic Theory
Exam 2: Atoms, Molecules, and Ions61 Questions
Exam 3: Stoichiometry100 Questions
Exam 4: Chemical Reactions and Solutions Stoichiometry93 Questions
Exam 5: Gases113 Questions
Exam 6: Chemical Equilibrium71 Questions
Exam 7: Acids and Bases119 Questions
Exam 8: Applications of Aqueous Equilibria171 Questions
Exam 9: Energy, Enthalpy, and Thermochemistry81 Questions
Exam 10: Spontaneity, Entropy, and Free Energy138 Questions
Exam 11: Electrochemistry85 Questions
Exam 12: Quantum Mechanics and Atomic Theory120 Questions
Exam 13: Bonding: General Concepts135 Questions
Exam 14: Covalent Bonding: Orbitals76 Questions
Exam 15: Chemical Kinetics119 Questions
Exam 16: Liquids and Solids106 Questions
Exam 17: Properties of Solutions99 Questions
Exam 18: The Representative Elements122 Questions
Exam 19: Transition Metals and Coordination Chemistry91 Questions
Exam 20: The Nucleus: a Chemists View68 Questions
Exam 21: Organic and Biochemical Molecules118 Questions
Select questions type
Given the electron configurations of the following neutral atoms, identify the element, and state the number of unpaired electrons in its ground state.
-[Ar] 4s23d7
(Essay)
4.8/5  (40)
(40)
In which groups do all the elements have the same number of valence electrons?
(Multiple Choice)
4.8/5  (35)
(35)
Which of the following combinations of quantum numbers do not represent permissible solutions of the Schrodinger equation for the electron in the hydrogen atom? (In other words, which combination of quantum numbers is not allowed?) (Combinations are listed as follows: n, l, m(l), m(s).)
(Multiple Choice)
4.8/5  (43)
(43)
From the following list of observations, choose the one that most clearly supports the conclusion that atoms contain electrons.
(Multiple Choice)
4.9/5  (27)
(27)
Which two elements are most likely to have the same oxidation state in an ionic compound?
(Multiple Choice)
4.8/5  (29)
(29)
what will be the kinetic energy of the ejected electrons? h = 6.626 x 10-34J.s, c = 2.998 x 108 m/s
(Multiple Choice)
4.9/5  (28)
(28)
Which form of electromagnetic radiation has the shortest wavelengths?
(Multiple Choice)
4.8/5  (38)
(38)
Consider the following sets of quantum numbers. Which set(s) represent(s) impossible combinations? 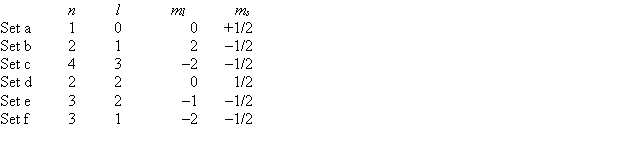
(Essay)
4.7/5  (32)
(32)
Which statements about hydrogen are true?
I. H has a lower ionization energy than He.
II. H- is smaller than H.
III. H has a higher effective nuclear charge than He.
IV. H is always a metal.
V. H does not have a second ionization energy.
(Multiple Choice)
4.9/5  (36)
(36)
Place the elements As, S, and Ar in order of increasing atomic radius.
(Multiple Choice)
4.7/5  (25)
(25)
Researchers recently formed a new synthetic element with atomic number 109. Which of the following elements would have chemical properties most similar to this new element?
(Multiple Choice)
4.7/5  (37)
(37)
The statement that the first ionization energy for an oxygen atom is lower than the first ionization energy for a nitrogen atom is
(Multiple Choice)
4.8/5  (35)
(35)
An element has the electron configuration [Kr] 4d105s25p2. The element is a(n)
(Multiple Choice)
4.7/5  (43)
(43)
Which of the following atoms has 3 electrons in p orbitals in its valence shell?
(Multiple Choice)
4.8/5  (39)
(39)
From the following list of observations, choose the one that most clearly supports the conclusion that electrons have wave properties.
(Multiple Choice)
4.8/5  (39)
(39)
What is the electron configuration of the element with atomic number 113?
(Multiple Choice)
4.8/5  (34)
(34)
What is the wavelength, in nanometers, of a photon of light whose frequency is 5.38 *1014 Hz?
(Multiple Choice)
4.8/5  (37)
(37)
Showing 81 - 100 of 120
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)