Exam 12: Quantum Mechanics and Atomic Theory
Exam 2: Atoms, Molecules, and Ions61 Questions
Exam 3: Stoichiometry100 Questions
Exam 4: Chemical Reactions and Solutions Stoichiometry93 Questions
Exam 5: Gases113 Questions
Exam 6: Chemical Equilibrium71 Questions
Exam 7: Acids and Bases119 Questions
Exam 8: Applications of Aqueous Equilibria171 Questions
Exam 9: Energy, Enthalpy, and Thermochemistry81 Questions
Exam 10: Spontaneity, Entropy, and Free Energy138 Questions
Exam 11: Electrochemistry85 Questions
Exam 12: Quantum Mechanics and Atomic Theory120 Questions
Exam 13: Bonding: General Concepts135 Questions
Exam 14: Covalent Bonding: Orbitals76 Questions
Exam 15: Chemical Kinetics119 Questions
Exam 16: Liquids and Solids106 Questions
Exam 17: Properties of Solutions99 Questions
Exam 18: The Representative Elements122 Questions
Exam 19: Transition Metals and Coordination Chemistry91 Questions
Exam 20: The Nucleus: a Chemists View68 Questions
Exam 21: Organic and Biochemical Molecules118 Questions
Select questions type
The energy equation for a particle in a cubic box of dimensions Lx = Ly = Lz is
Enx, ny, nz = 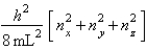 Assume that 8 electrons occupy the allowed energy levels and that 2 electrons can occupy each allowed energy level.
A)In the ground state, how many of the 8 electrons have energy equal to
Assume that 8 electrons occupy the allowed energy levels and that 2 electrons can occupy each allowed energy level.
A)In the ground state, how many of the 8 electrons have energy equal to 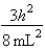 ?
B)In the ground state, how many of the 8 electrons have energy equal to
?
B)In the ground state, how many of the 8 electrons have energy equal to 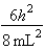 ?
C)In the ground state, how many of the 8 electrons have energy equal to
?
C)In the ground state, how many of the 8 electrons have energy equal to 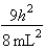 ?
D)Calculate the wavelength of light necessary to promote the highest-energy ground-state electron into the lowest-energy excited state. Assume a cubic box with dimensions 1.50 nm * 1.50 nm * 1.50 nm.
?
D)Calculate the wavelength of light necessary to promote the highest-energy ground-state electron into the lowest-energy excited state. Assume a cubic box with dimensions 1.50 nm * 1.50 nm * 1.50 nm.
(Short Answer)
4.7/5  (25)
(25)
From the following list of observations, choose the one that most clearly supports the conclusion that electromagnetic radiation has wave characteristics.
(Multiple Choice)
4.9/5  (34)
(34)
Given the electron configurations of the following neutral atoms, identify the element, and state the number of unpaired electrons in its ground state.
-[Xe] 4f146s25d2
(Essay)
4.8/5  (36)
(36)
How many electrons can be described by the quantum numbers n = 3, l = 1?
(Multiple Choice)
4.8/5  (27)
(27)
Which of the following statements is true about the ionization energy of Mg+?
(Multiple Choice)
4.9/5  (27)
(27)
Consider the following orderings.
I. Al < Si < P < Cl
II. Be < Mg < Ca < Sr
III. I < Br < Cl < F
IV. Na+ < Mg2+ < Al3+ < Si4+
-Which of these give(s) a correct trend in ionization energy?
(Multiple Choice)
4.8/5  (32)
(32)
Nitrogen has 5 valence electrons. Consider the following electron arrangements.
-Which represents the ground state for N?
(Multiple Choice)
4.9/5  (46)
(46)
How many electrons can be contained in all of the orbitals with n = 4?
(Multiple Choice)
4.7/5  (30)
(30)
From the following list of observations, choose the one that most clearly supports the conclusion of de Broglie wavelengths.
(Multiple Choice)
4.9/5  (36)
(36)
A photographic film needs a minimum of 80.0 kJ/mol for exposure. What is the longest wavelength of radiation with sufficient energy to expose the film?
(Multiple Choice)
4.9/5  (30)
(30)
Which is the highest occupied energy orbital in a silicon atom?
(Multiple Choice)
4.8/5  (33)
(33)
The energy expressions for the electrons in the He+ ion and the hydrogen atom are En (H) = -a/n2 and En (He+) = -4a/n2
Which of the following statements is(are) correct?
I. For the transitions 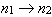 , the frequency is larger for H than for He+.
II. The first ionization energy of the H atom is smaller than the second.
III. The 1s orbital in He+ is larger (in the sense that the probability density is
shifted outward) than the 1s orbital in H.
, the frequency is larger for H than for He+.
II. The first ionization energy of the H atom is smaller than the second.
III. The 1s orbital in He+ is larger (in the sense that the probability density is
shifted outward) than the 1s orbital in H.
(Multiple Choice)
4.7/5  (39)
(39)
Given the electron configurations of the following neutral atoms, identify the element, and state the number of unpaired electrons in its ground state.
-[Kr] 4d105s25p1
(Essay)
4.7/5  (42)
(42)
An electron in a one-dimensional box requires energy with wavelength 8080 nm to excite it from the n = 2 energy level to the n = 3 energy level. Calculate the length of the box.
(Multiple Choice)
4.8/5  (30)
(30)
The first ionization energy of Mg is 735 kJ/mol. Calculate Zeff.
(Multiple Choice)
5.0/5  (41)
(41)
Which of the following statements is(are) true?
I. An excited atom can return to its ground state by absorbing electromagnetic radiation.
II. The energy of an atom is increased when electromagnetic radiation is emitted from it.
III. The energy of electromagnetic radiation increases as its frequency increases.
IV. An electron in the n = 4 state in the hydrogen atom can go to the n = 2 state by emitting electromagnetic radiation at the appropriate frequency.
V. The frequency and wavelength of electromagnetic radiation are inversely proportional to each other.
(Multiple Choice)
4.8/5  (33)
(33)
Showing 41 - 60 of 120
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)