Exam 12: Quantum Mechanics and Atomic Theory
Exam 2: Atoms, Molecules, and Ions61 Questions
Exam 3: Stoichiometry100 Questions
Exam 4: Chemical Reactions and Solutions Stoichiometry93 Questions
Exam 5: Gases113 Questions
Exam 6: Chemical Equilibrium71 Questions
Exam 7: Acids and Bases119 Questions
Exam 8: Applications of Aqueous Equilibria171 Questions
Exam 9: Energy, Enthalpy, and Thermochemistry81 Questions
Exam 10: Spontaneity, Entropy, and Free Energy138 Questions
Exam 11: Electrochemistry85 Questions
Exam 12: Quantum Mechanics and Atomic Theory120 Questions
Exam 13: Bonding: General Concepts135 Questions
Exam 14: Covalent Bonding: Orbitals76 Questions
Exam 15: Chemical Kinetics119 Questions
Exam 16: Liquids and Solids106 Questions
Exam 17: Properties of Solutions99 Questions
Exam 18: The Representative Elements122 Questions
Exam 19: Transition Metals and Coordination Chemistry91 Questions
Exam 20: The Nucleus: a Chemists View68 Questions
Exam 21: Organic and Biochemical Molecules118 Questions
Select questions type
Nitrogen has 5 valence electrons. Consider the following electron arrangements.
-Which represents the ground state for the N- ion?
(Multiple Choice)
4.7/5  (33)
(33)
How many valence electrons do the alkaline earth metals have?
(Multiple Choice)
4.9/5  (35)
(35)
An electron in a 10.0-nm one-dimensional box is excited from the ground state into a higher energy state by absorbing a photon with wavelength 1.374 *10-5 m. Determine the final energy level for this transition.
(Multiple Choice)
4.7/5  (32)
(32)
An element E has the electron configuration [Ar]3d104s24p3. What is the formula for the fluoride of E most likely to be?
(Multiple Choice)
4.7/5  (38)
(38)
What is the probability of finding a particle in a one-dimensional box in energy level n = 4 between x = L/4 and x = L/2? (L is the length of the box.)
(Multiple Choice)
4.8/5  (35)
(35)
For the set of elements Li, K, Ar, and Kr, which element has the largest atomic radius? Explain any deviation from the expected pattern.
(Essay)
4.7/5  (35)
(35)
How many unpaired electrons does arsenic have in its ground state?
(Multiple Choice)
4.9/5  (38)
(38)
For which of the following elements does the electron configuration for the lowest energy state show a partially filled d orbital?
(Multiple Choice)
4.9/5  (36)
(36)
From the following list of observations, choose the one that most clearly supports the conclusion that the mass of the atom is located mainly in the nucleus.
(Multiple Choice)
4.9/5  (34)
(34)
How many electrons can be described by the quantum numbers n = 4, l = 3, ml = -1, ms = -1?
(Multiple Choice)
4.8/5  (35)
(35)
Of the following elements, which needs 3 electrons to complete its valence shell?
(Multiple Choice)
4.9/5  (40)
(40)
What is the wavelength of light that is emitted when an excited electron in the hydrogen atom falls from the n = 5 level to the n = 2 level?
(Multiple Choice)
4.8/5  (36)
(36)
The valence electron configuration of an element is ns2(n - 1)d10np2. To which group does X belong?
(Multiple Choice)
4.8/5  (30)
(30)
How many unpaired electrons does chlorine have in its ground state?
(Multiple Choice)
4.8/5  (27)
(27)
The energy equation for a particle in a cubic box of dimensions Lx = Ly = Lz is Enx, ny, nz = 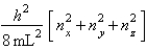 For a particle in a cubic box, how many degenerate energy levels have energy equal to 14 h2/8 mL2?
For a particle in a cubic box, how many degenerate energy levels have energy equal to 14 h2/8 mL2?
(Multiple Choice)
4.8/5  (38)
(38)
For an electron in a 2.00-nm one-dimensional box, calculate the wavelength of electromagnetic radiation to excite the electron from the ground state to the level with n = 3.
(Multiple Choice)
4.9/5  (35)
(35)
How many unpaired electrons are there in an atom of sulfur in its ground state?
(Multiple Choice)
4.9/5  (30)
(30)
Consider the following orderings.
I. Al < Si < P < Cl
II. Be < Mg < Ca < Sr
III. I < Br < Cl < F
IV. Na+ < Mg2+ < Al3+ < Si4+
-Which of the following exhibits the correct orders (increasing) for atomic radius and ionization energy, respectively?
(Multiple Choice)
4.7/5  (34)
(34)
How many unpaired electrons does manganese have in its ground state?
(Multiple Choice)
4.8/5  (34)
(34)
Showing 101 - 120 of 120
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)