Exam 8: Applications of Aqueous Equilibria
Exam 2: Atoms, Molecules, and Ions66 Questions
Exam 3: Stoichiometry105 Questions
Exam 4: Types of Chemical Reactions and Solution Stoichiometry98 Questions
Exam 5: Gases118 Questions
Exam 6: Chemical Equilibrium78 Questions
Exam 7: Acids and Bases126 Questions
Exam 8: Applications of Aqueous Equilibria177 Questions
Exam 9: Energy, Enthalpy, and Thermochemistry86 Questions
Exam 10: Spontaneity, Entropy, and Free Energy143 Questions
Exam 11: Electrochemistry90 Questions
Exam 12: Quantum Mechanics and Atomic Theory125 Questions
Exam 13: Bonding: General Concepts136 Questions
Exam 14: Covalent Bonding: Orbitals81 Questions
Exam 15: Chemical Kinetics124 Questions
Exam 16: Liquids and Solids111 Questions
Exam 17: Properties of Solutions105 Questions
Exam 18: The Representative Elements127 Questions
Exam 19: Transition Metals and Coordination Chemistry96 Questions
Exam 20: The Nucleus: a Chemists View73 Questions
Exam 21: Organic and Biochemical Molecules123 Questions
Select questions type
What combination of substances will give a buffered solution that has a pH of 5.05? Assume each pair of substances is dissolved in 5.0 L of water. (Kb for NH3 = 1.8 × 10-5; Kb for C5H5N = 1.7 × 10-9)
(Multiple Choice)
4.8/5  (41)
(41)
You are given 5.00 mL of an H2SO4 solution of unknown concentration. You divide the 5.00-mL sample into five 1.00-mL samples and titrate each separately with 0.1000 M NaOH. In each titration, the H2SO4 is completely neutralized. The average volume of NaOH solution used to reach the endpoint is 15.3 mL. What was the concentration of H2SO4 in the 5.00-mL sample?
(Multiple Choice)
4.9/5  (37)
(37)
A titration of 100.0 mL of 1.00 M malonic acid (H2A) was done with 1.00 M NaOH. For malonic acid, Ka1 = 1.49 × 10-2, Ka2 = 2.03 × 10-6.
-Calculate the [H+] after 50.00 mL of 1.00 M NaOH has been added.
(Multiple Choice)
4.8/5  (42)
(42)
Which of the following solutions will be the best buffer at a pH of 9.26? (Ka for HC2H3O2 is 1.8 × 10-5; Kb for NH3 is 1.8 × 10-5.)
(Multiple Choice)
4.9/5  (35)
(35)
Given the following Ksp values 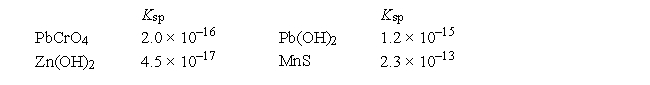 which statement about solubility, in moles per liter, in water is correct?
which statement about solubility, in moles per liter, in water is correct?
(Multiple Choice)
4.8/5  (39)
(39)
A 73.5-mL sample of 0.18 M HNO2 (Ka = 4.0 × 10-4) is titrated with 0.12 M NaOH. What is the pH after 25.2 mL of NaOH has been added?
(Multiple Choice)
4.9/5  (42)
(42)
The Ag+ ion reacts with NH3 to form the following complex ions: 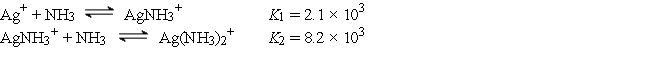 AgCl (Ksp = 1.6 × 10-10) is dissolved to its solubility limit in 10.0 M NH3. Calculate the equilibrium concentrations of Ag+, Cl-, Ag(NH3)2+, and NH3.
AgCl (Ksp = 1.6 × 10-10) is dissolved to its solubility limit in 10.0 M NH3. Calculate the equilibrium concentrations of Ag+, Cl-, Ag(NH3)2+, and NH3.
(Essay)
4.9/5  (28)
(28)
In the titration of a weak acid HA with 0.100 M NaOH, the stoichiometric point is known to occur at a pH value of approximately 11. Which of the following indicators would be best to use to mark the endpoint of this titration?
(Multiple Choice)
4.9/5  (36)
(36)
The salt AgCl is ________ soluble in strong acid solution than in water.
(Multiple Choice)
4.9/5  (36)
(36)
The solubility, in moles per liter, of Ag2CrO4 is 1.3 × 10-4 M at 25°C. Calculate Ksp for this compound.
(Multiple Choice)
4.8/5  (42)
(42)
Which of the following statements is/are true of ligands?
1) A ligand is a molecule or an ion having a lone pair of electrons that can be donated to the metal ion to form a covalent bond.2. A complex ion is a charged species consisting of a metal ion surrounded by ligands.
3) The number of ligands attached to a metal ion is called the coordination number.
(Multiple Choice)
4.7/5  (41)
(41)
What is the molar solubility of AgCl (Ksp = 1.6 × 10-10) in 0.0020 M sodium chloride at 25°C?
(Multiple Choice)
4.8/5  (35)
(35)
Consider a solution of 2.0 M HCN and 1.0 M NaCN (Ka for HCN = 6.2 × 10-10). Which of the following statements is true?
(Multiple Choice)
4.9/5  (36)
(36)
If 30 mL of 5.0 × 10-4 M Ca(NO3)2 is added to 70 mL of 2.0 × 10-4 M NaF, will a precipitate form? (Ksp of CaF2 = 4.0 × 10-11)
(Multiple Choice)
4.8/5  (35)
(35)
A 100.-mL sample of 0.10 M HCl is mixed with 50. mL of 0.10 M NH3. What is the resulting pH? (Kb for NH3 = 1.8 × 10-5)
(Multiple Choice)
4.7/5  (34)
(34)
Consider the titration of 200.0 mL of a 0.100 M solution of the weak acid H2A with 0.200 M NaOH. The first equivalence point is reached after 100.0 mL of 0.200 M NaOH has been added, and the pH is 6.27. The pH after 65.0 mL of 0.200 M NaOH has been added, the pH is 4.95.Calculate the value of Ka1 for H2A.
(Multiple Choice)
4.8/5  (35)
(35)
Calculate the concentration of chromate ion, CrO42-, in a saturated solution of CaCrO4 (Ksp = 7.1 × 10-4).
(Multiple Choice)
4.8/5  (39)
(39)
Showing 161 - 177 of 177
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)