Exam 8: Applications of Aqueous Equilibria
Exam 2: Atoms, Molecules, and Ions66 Questions
Exam 3: Stoichiometry105 Questions
Exam 4: Types of Chemical Reactions and Solution Stoichiometry98 Questions
Exam 5: Gases118 Questions
Exam 6: Chemical Equilibrium78 Questions
Exam 7: Acids and Bases126 Questions
Exam 8: Applications of Aqueous Equilibria177 Questions
Exam 9: Energy, Enthalpy, and Thermochemistry86 Questions
Exam 10: Spontaneity, Entropy, and Free Energy143 Questions
Exam 11: Electrochemistry90 Questions
Exam 12: Quantum Mechanics and Atomic Theory125 Questions
Exam 13: Bonding: General Concepts136 Questions
Exam 14: Covalent Bonding: Orbitals81 Questions
Exam 15: Chemical Kinetics124 Questions
Exam 16: Liquids and Solids111 Questions
Exam 17: Properties of Solutions105 Questions
Exam 18: The Representative Elements127 Questions
Exam 19: Transition Metals and Coordination Chemistry96 Questions
Exam 20: The Nucleus: a Chemists View73 Questions
Exam 21: Organic and Biochemical Molecules123 Questions
Select questions type
What is the molarity of a sodium hydroxide solution if 25.0 mL of this solution reacts exactly with 22.30 mL of 0.253 M sulfuric acid?
(Multiple Choice)
4.9/5  (47)
(47)
You are given a solution of the weak base Novocain, Nvc. Its pH is 11.00. You add to the solution a small amount of a salt containing the conjugate acid of Novocain, NvcH+. Which statement is true?
(Multiple Choice)
4.9/5  (42)
(42)
A titration of 100.0 mL of 1.00 M malonic acid (H2A) was done with 1.00 M NaOH. For malonic acid, Ka1 = 1.49 × 10-2, Ka2 = 2.03 × 10-6.
-Calculate [H+] after 300.0 mL of 1.00 M NaOH has been added.
(Multiple Choice)
4.8/5  (41)
(41)
Calculate the pH when 200.0 mL of a 1.00 M solution of H2A (Ka1 = 1.0 × 10-6, Ka2 = 1.0 × 10-10) is titrated with the following volumes of 1.00 M NaOH.
-0 mL of 1.00 M NaOH
(Multiple Choice)
4.8/5  (37)
(37)
Consider the titration of 100.0 mL of 0.250 M aniline (Kb = 3.8 × 10-10) with 0.500 M HCl. Calculate the pH of the solution at the stoichiometric point.
(Multiple Choice)
4.9/5  (35)
(35)
Calculate the concentration of [H+] of a buffered solution containing 5.0 × 10−4 M HCN (Ka = 6.2 × 10−10) and 1.5 × 10−4 M NaCN.
(Multiple Choice)
4.9/5  (43)
(43)
A 50.0-mL sample of 2.0 × 10-4 M CuNO3 is added to 50.0 mL of 4.0 M NaCN. Cu+ reacts with CN- to form the complex ion Cu(CN)32-: 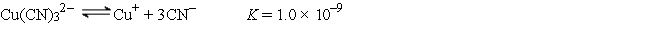 Calculate the solubility of CuBr(s) (Ksp = 1.0 × 10-5) in 1.0 L of 1.0 M NaCN.
Calculate the solubility of CuBr(s) (Ksp = 1.0 × 10-5) in 1.0 L of 1.0 M NaCN.
(Multiple Choice)
4.9/5  (41)
(41)
Differentiate between the equivalence point and the endpoint in an acid-base titration.
(Short Answer)
4.8/5  (43)
(43)
A 200-mL solution contains 0.018 mol each of I-, Br-, and Cl-. When the solution is mixed with 200 mL of 0.24 M AgNO3, how much AgCl(s) precipitates out? 
(Multiple Choice)
4.9/5  (40)
(40)
Calculate the pH when 200.0 mL of a 1.00 M solution of H2A (Ka1 = 1.0 × 10-6, Ka2 = 1.0 × 10-10) is titrated with the following volumes of 1.00 M NaOH.
-200.0 mL of 1.00 M NaOH
(Multiple Choice)
4.7/5  (41)
(41)
Which of the following salts shows the lowest solubility in water? Ksp values are as follows:
Ag2S = 1.6 × 10-49; Bi2S3 = 1.0 × 10-72; HgS = 1.6 × 10-54; Mg(OH)2 = 8.9 × 10-12; MnS = 2.3 × 10-13)
(Multiple Choice)
4.8/5  (37)
(37)
You have solutions of 0.200 M HNO2 and 0.200 M KNO2 (Ka for HNO2 = 4.00 × 10-4). A buffer of pH 3.000 is needed. What volumes of HNO2 and KNO2 are required to make 1 L of buffered solution?
(Multiple Choice)
4.9/5  (46)
(46)
A 0.012-mol sample of Na2SO4 is added to 400 mL of each of two solutions. One solution contains 1.5 × 10-3 M BaCl2; the other contains 1.5 × 10-3 M CaCl2. Ksp for BaSO4 = 1.5 × 10-9 and Ksp for CaSO4 = 6.1 × 10-5. Which of the following statements is true?
(Multiple Choice)
4.8/5  (34)
(34)
A 200.0-mL sample of the weak acid H3A (0.100 M) is titrated with 0.200 M NaOH. What are the major species at each of the following points in the titration? (Water is always assumed to be a major species.)
-After 300.0 mL of 0.200 M NaOH is added
(Multiple Choice)
4.9/5  (35)
(35)
Consider the following information about the diprotic acid ascorbic acid (H2As for short, molar mass = 176.1).  The titration curve for disodium ascorbate, Na2As, with standard HCl is shown below:
The titration curve for disodium ascorbate, Na2As, with standard HCl is shown below: 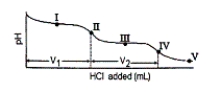 -What is the pH at point I (V1/2 HCl added)?
-What is the pH at point I (V1/2 HCl added)?
(Multiple Choice)
4.9/5  (39)
(39)
Calculate the pH when 200.0 mL of a 1.00 M solution of H2A (Ka1 = 1.0 × 10-6, Ka2 = 1.0 × 10-10) is titrated with the following volumes of 1.00 M NaOH.
-300.0 mL of 1.00 M NaOH
(Multiple Choice)
4.8/5  (37)
(37)
The contents of the flask are transferred quantitatively to a 2L volumetric. 100ml of 1 M HCL is added and the flask filled to the mark with water. There is still observable solid calcium hydroxide in the bottom of the new flask. What is the pH of this solution?
(Multiple Choice)
4.8/5  (32)
(32)
A 50.00-mL sample of 0.100 M Ca(NO3)2 is mixed with 50.00 mL of 0.200 M NaF. When the system has come to equilibrium, which of the following sets of conditions will hold? The Ksp for CaF2 is 4.0 × 10-11. 
(Multiple Choice)
4.9/5  (30)
(30)
In the titration of 100.0 mL of a 0.200 M solution of H2A (Ka1 = 1.0 × 10-5, Ka2 = 1.0 × 10-8), what volume of 0.400 M NaOH must be added to reach a pH of 5.00?
(Multiple Choice)
4.8/5  (33)
(33)
One milliliter (1.00 mL) of acid taken from a lead storage battery is pipetted into a flask. Water and phenolphthalein indicator are added, and the solution is titrated with 0.38 M NaOH until a pink color appears; 15.8 mL is required. Find, to within 5%, the number of grams of H2SO4 (formula weight = 98) present in 1 L of the battery acid.
(Multiple Choice)
4.8/5  (48)
(48)
Showing 61 - 80 of 177
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)