Exam 8: Applications of Aqueous Equilibria
Exam 2: Atoms, Molecules, and Ions66 Questions
Exam 3: Stoichiometry105 Questions
Exam 4: Types of Chemical Reactions and Solution Stoichiometry98 Questions
Exam 5: Gases118 Questions
Exam 6: Chemical Equilibrium78 Questions
Exam 7: Acids and Bases126 Questions
Exam 8: Applications of Aqueous Equilibria177 Questions
Exam 9: Energy, Enthalpy, and Thermochemistry86 Questions
Exam 10: Spontaneity, Entropy, and Free Energy143 Questions
Exam 11: Electrochemistry90 Questions
Exam 12: Quantum Mechanics and Atomic Theory125 Questions
Exam 13: Bonding: General Concepts136 Questions
Exam 14: Covalent Bonding: Orbitals81 Questions
Exam 15: Chemical Kinetics124 Questions
Exam 16: Liquids and Solids111 Questions
Exam 17: Properties of Solutions105 Questions
Exam 18: The Representative Elements127 Questions
Exam 19: Transition Metals and Coordination Chemistry96 Questions
Exam 20: The Nucleus: a Chemists View73 Questions
Exam 21: Organic and Biochemical Molecules123 Questions
Select questions type
Consider the following information about the diprotic acid ascorbic acid (H2As for short, molar mass = 176.1).  The titration curve for disodium ascorbate, Na2As, with standard HCl is shown below:
The titration curve for disodium ascorbate, Na2As, with standard HCl is shown below: 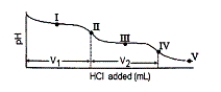 -What major species is(are) present at point III?
-What major species is(are) present at point III?
(Multiple Choice)
4.9/5  (36)
(36)
You have two salts, AgX and AgY, with very similar Ksp values. You know that Ka for HX is much greater than Ka for HY. Which salt is more soluble in acidic solution?
(Multiple Choice)
4.9/5  (34)
(34)
Given the following values of equilibrium constants: 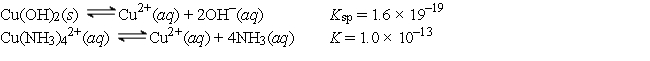 What is the value of the equilibrium constant for the following reaction?
Cu(OH)2(s) + 4NH3(aq)
What is the value of the equilibrium constant for the following reaction?
Cu(OH)2(s) + 4NH3(aq)  Cu(NH3)42+(aq) + 2OH-(aq)
Cu(NH3)42+(aq) + 2OH-(aq)
(Multiple Choice)
4.8/5  (29)
(29)
Sodium chloride is added slowly to a solution that is 0.010 M in Cu+, Ag+, and Au+. The Ksp values for the chloride salts are 1.9 × 10-7, 1.6 × 10-10, and 2.0 × 10-13, respectively. Which compound will precipitate first?
(Multiple Choice)
4.8/5  (37)
(37)
What quantity of NaOH(s) must be added to 1.00 L of 0.200 M HCl to achieve a pH of 12.00? (Assume no volume change.)
(Multiple Choice)
4.7/5  (35)
(35)
Methyl orange is an indicator with a Ka of 1 × 10-4. Its acid form, HIn, is red, while its base form, In-, is yellow. At pH 6.0, the indicator will be
(Multiple Choice)
4.8/5  (38)
(38)
A solution containing 10. mmol of CO32- and 5.0 mmol of HCO3- is titrated with
1)0 M HCl.What total volume of HCl must be added to reach the second equivalence point?
(Multiple Choice)
4.9/5  (50)
(50)
Equal volumes of 0.1 M HCl and 0.1 M HC2H3O2 are titrated with 0.1 M NaOH. Which of the following would be equal for both titrations?
(Multiple Choice)
4.8/5  (31)
(31)
For carbonic acid (H2CO3), Ka1 = 4.30 × 10-7 and Ka2 = 5.62 × 10-11. Calculate the pH of a 0.50 M solution of Na2CO3.
(Multiple Choice)
4.9/5  (48)
(48)
A solution is formed by mixing 50.0 mL of 10.00 M NaCN with 50.0 mL of 2.0 × 10-3 M CuNO3. Cu(I) forms complex ions with cyanide as follows: ![A solution is formed by mixing 50.0 mL of 10.00 M NaCN with 50.0 mL of 2.0 × 10<sup>-3</sup> M CuNO<sub>3</sub>. Cu(I) forms complex ions with cyanide as follows: Calculate the following concentrations at equilibrium: -[Cu<sup>+</sup>]](https://storage.examlex.com/TB6422/11eaaf91_9e28_6ed1_892c_81ba02cfc46a_TB6422_00_TB6422_00_TB6422_00.jpg) Calculate the following concentrations at equilibrium:
-[Cu+]
Calculate the following concentrations at equilibrium:
-[Cu+]
(Multiple Choice)
4.8/5  (36)
(36)
A 200.0-mL sample of the weak acid H3A (0.100 M) is titrated with 0.200 M NaOH. What are the major species at each of the following points in the titration? (Water is always assumed to be a major species.)
-After 350.0 mL of 0.200 M NaOH is added
(Multiple Choice)
4.9/5  (37)
(37)
A solution is formed by mixing 50.0 mL of 10.00 M NaCN with 50.0 mL of 2.0 × 10-3 M CuNO3. Cu(I) forms complex ions with cyanide as follows: ![A solution is formed by mixing 50.0 mL of 10.00 M NaCN with 50.0 mL of 2.0 × 10<sup>-3</sup> M CuNO<sub>3</sub>. Cu(I) forms complex ions with cyanide as follows: Calculate the following concentrations at equilibrium: -[Cu(CN)<sub>2</sub><sup>-</sup>]](https://storage.examlex.com/TB6422/11eaaf91_9e28_6ed1_892c_81ba02cfc46a_TB6422_00_TB6422_00_TB6422_00.jpg) Calculate the following concentrations at equilibrium:
-[Cu(CN)2-]
Calculate the following concentrations at equilibrium:
-[Cu(CN)2-]
(Multiple Choice)
4.8/5  (25)
(25)
In the titration of 250.0 mL of 0.20 M H3PO4 with 0.10 M NaOH, the pH of the solution after the addition of some NaOH is 4.66. Which of the following phosphate-containing species is present in the largest amount? For H3PO4, Ka1 = 7.5 × 10-3, Ka2 = 6.2 × 10-8, and Ka3 = 4.8 × 10-13.
(Multiple Choice)
4.8/5  (42)
(42)
The overall Kf for the complex ion Ag(NH3)2+ is 1.7 × 107. Ksp for AgI is 1.5 × 10-16. What is the molar solubility of AgI in a solution that is 2.0 M in NH3?
(Multiple Choice)
4.9/5  (37)
(37)
How many moles of CaF2 will dissolve in 3.0 L of 0.050 M NaF solution? (Ksp for CaF2 = 4.0 × 10-11)
(Multiple Choice)
4.8/5  (45)
(45)
The concentration of Mg2+ in seawater is 0.052 M. At what pH will 99% of the Mg2+ be precipitated as the hydroxide? (Ksp for Mg(OH)2 = 8.9 × 10-12)
(Multiple Choice)
4.9/5  (43)
(43)
The solubility of Fe(OH)2 in water is 7.9 × 10-6 mol/L at 25° C. What is Ksp for Fe(OH)2 at 25° C?
(Multiple Choice)
4.8/5  (39)
(39)
The two salts AgX and AgY have very similar solubilities in water. The salt AgX is much more soluble in acid than is AgY. What can be said about the relative strengths of the acids HX and HY?
(Multiple Choice)
4.8/5  (46)
(46)
How much solid NaCN must be added to 1.0 L of a 0.5 M HCN solution to produce a solution with pH 7.0? Ka = 6.2 × 10-10 for HCN.
(Multiple Choice)
4.9/5  (33)
(33)
Calculate the pH when 200.0 mL of a 1.00 M solution of H2A (Ka1 = 1.0 × 10-6, Ka2 = 1.0 × 10-10) is titrated with the following volumes of 1.00 M NaOH.100.0 mL of 1.00 M NaOH
(Multiple Choice)
4.8/5  (31)
(31)
Showing 21 - 40 of 177
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)