Exam 8: Applications of Aqueous Equilibria
Exam 2: Atoms, Molecules, and Ions66 Questions
Exam 3: Stoichiometry105 Questions
Exam 4: Types of Chemical Reactions and Solution Stoichiometry98 Questions
Exam 5: Gases118 Questions
Exam 6: Chemical Equilibrium78 Questions
Exam 7: Acids and Bases126 Questions
Exam 8: Applications of Aqueous Equilibria177 Questions
Exam 9: Energy, Enthalpy, and Thermochemistry86 Questions
Exam 10: Spontaneity, Entropy, and Free Energy143 Questions
Exam 11: Electrochemistry90 Questions
Exam 12: Quantum Mechanics and Atomic Theory125 Questions
Exam 13: Bonding: General Concepts136 Questions
Exam 14: Covalent Bonding: Orbitals81 Questions
Exam 15: Chemical Kinetics124 Questions
Exam 16: Liquids and Solids111 Questions
Exam 17: Properties of Solutions105 Questions
Exam 18: The Representative Elements127 Questions
Exam 19: Transition Metals and Coordination Chemistry96 Questions
Exam 20: The Nucleus: a Chemists View73 Questions
Exam 21: Organic and Biochemical Molecules123 Questions
Select questions type
Consider a solution consisting of the following two buffer systems:
H2CO3  HCO3- + H+ pKa = 6.4
H2PO4-
HCO3- + H+ pKa = 6.4
H2PO4-  HPO42- + H+ pKa = 7.2
At pH 6.4, which one of the following is true of the relative amounts of acid and conjugate base present?
HPO42- + H+ pKa = 7.2
At pH 6.4, which one of the following is true of the relative amounts of acid and conjugate base present?
(Multiple Choice)
5.0/5  (36)
(36)
Calculate the molar concentration of uncomplexed Zn2+ in a solution that contains 0.20 mol of Zn(NH3)42+ per liter. The overall Kf for Zn(NH3)42+ is 3.8 × 109.
(Multiple Choice)
5.0/5  (33)
(33)
How many moles of Fe(OH)2 [Ksp = 1.8 × 10-15] will dissolve in 1 L of water buffered at pH = 12.00?
(Multiple Choice)
4.8/5  (31)
(31)
The concentration of Al3+ in a saturated solution of Al(OH)3 at 25°C is 5.2 × 10-9 M. Calculate the Ksp for Al(OH)3.
(Multiple Choice)
4.8/5  (34)
(34)
Calculate the pH of the final solution obtained by mixing the following solutions. For HCN, Ka = 6.2 × 10-10.50.0 mL of 0.10 M HNO3
60.0 mL of 0.20 M Ba(OH)2
95.0 mL of 0.20 M HClO4
195.0 mL of 1.0 × 10-4 M HCN
(Short Answer)
4.8/5  (30)
(30)
A 200.0-mL sample of the weak acid H3A (0.100 M) is titrated with 0.200 M NaOH. What are the major species at each of the following points in the titration? (Water is always assumed to be a major species.)
-After 220.0 mL of 0.200 M NaOH is added
(Multiple Choice)
4.8/5  (38)
(38)
Calculate the pH of a solution that contains 3.25 M HCN (Ka = 6.2 × 10-10), 1.00 M NaOH and 1.50 M NaCN.
(Multiple Choice)
4.8/5  (36)
(36)
A 50.0-mL sample of 2.0 × 10-4 M CuNO3 is added to 50.0 mL of 4.0 M NaCN. Cu+ reacts with CN- to form the complex ion Cu(CN)32-: 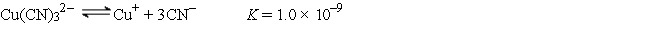 The concentration of Cu+ at equilibrium is
The concentration of Cu+ at equilibrium is
(Multiple Choice)
4.8/5  (36)
(36)
Consider a solution made by mixing 500.0 mL of 4.0 M NH3 and 500.0 mL of 0.40 M AgNO3. Ag+ reacts with NH3 to form AgNH3+ and Ag(NH3)2+: 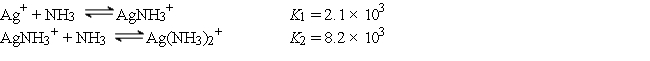 The concentration of Ag+ at equilibrium is
The concentration of Ag+ at equilibrium is
(Multiple Choice)
4.9/5  (49)
(49)
A titration of 100.0 mL of 1.00 M malonic acid (H2A) was done with 1.00 M NaOH. For malonic acid, Ka1 = 1.49 × 10-2, Ka2 = 2.03 × 10-6.
-Calculate [H+] after 150.0 mL of 1.00 M NaOH has been added.
(Multiple Choice)
4.9/5  (39)
(39)
In a solution prepared by adding excess PbI2(s) [Ksp = 1.4 × 10-8] to water, [I-] at equilibrium is
(Multiple Choice)
4.9/5  (31)
(31)
A 100.0-mL sample of 0.2 M (CH3)3N (Kb = 5.3 × 10-5) is titrated with 0.2 M HCl. What is the pH at the equivalence point?
(Multiple Choice)
4.7/5  (42)
(42)
After adding 25.0 mL of 0.100 M NaOH to 100.0 mL of 0.100 M weak acid (HA), the pH is found to be 5.90. Determine the value of Ka for the acid HA.
(Multiple Choice)
4.9/5  (29)
(29)
What is the solubility of Mg(OH)2 (Ksp = 8.9 × 10-12) in 1.0 L of a solution buffered (with large capacity) at pH 10.0?
(Multiple Choice)
4.8/5  (29)
(29)
Calculate the pH of a solution made by mixing 46.0 mL of 0.350 M NaA (Ka for HA = 1.0 × 10-9) with 26.0 mL of 0.190 M HCl.
(Multiple Choice)
4.8/5  (37)
(37)
A 50.0-mL sample of 2.0 × 10-4 M CuNO3 is added to 50.0 mL of 4.0 M NaCN. Cu+ reacts with CN- to form the complex ion Cu(CN)32-: 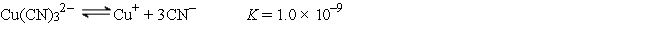 What is the concentration of CN- at equilibrium?
What is the concentration of CN- at equilibrium?
(Multiple Choice)
4.8/5  (27)
(27)
Chromate ion is added to a saturated solution of Ag2CrO4 to reach 0.10 M CrO42-. Calculate the final concentration of silver ion at equilibrium (Ksp for Ag2CrO4 is 9.0 × 10-12).
(Multiple Choice)
4.8/5  (41)
(41)
A student uses 16.60 mL of 0.100 M NaOH to titrate a 0.2000-g sample of an unknown acid. Which of the following acids is the unknown most likely to be? Assume that only the hydrogens bonded to oxygen are titrated by the sodium hydroxide and that, because of experimental error, the student's value may not be identical to the theoretical value.
(Multiple Choice)
4.9/5  (43)
(43)
The cation M2+ reacts with NH3 to form a series of complex ions as follows: 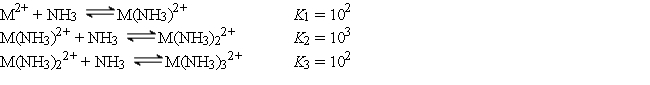 Consider an experiment in which 1.0 × 10-3 mol of M(NO3)2 is added to 1.0 L of 15.0 M NH3. Calculate the equilibrium concentrations of M2+, M(NH3)22+, and M(NH3)32+
Consider an experiment in which 1.0 × 10-3 mol of M(NO3)2 is added to 1.0 L of 15.0 M NH3. Calculate the equilibrium concentrations of M2+, M(NH3)22+, and M(NH3)32+
(Essay)
4.9/5  (39)
(39)
Showing 41 - 60 of 177
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)