Exam 8: Applications of Aqueous Equilibria
Exam 2: Atoms, Molecules, and Ions66 Questions
Exam 3: Stoichiometry105 Questions
Exam 4: Types of Chemical Reactions and Solution Stoichiometry98 Questions
Exam 5: Gases118 Questions
Exam 6: Chemical Equilibrium78 Questions
Exam 7: Acids and Bases126 Questions
Exam 8: Applications of Aqueous Equilibria177 Questions
Exam 9: Energy, Enthalpy, and Thermochemistry86 Questions
Exam 10: Spontaneity, Entropy, and Free Energy143 Questions
Exam 11: Electrochemistry90 Questions
Exam 12: Quantum Mechanics and Atomic Theory125 Questions
Exam 13: Bonding: General Concepts136 Questions
Exam 14: Covalent Bonding: Orbitals81 Questions
Exam 15: Chemical Kinetics124 Questions
Exam 16: Liquids and Solids111 Questions
Exam 17: Properties of Solutions105 Questions
Exam 18: The Representative Elements127 Questions
Exam 19: Transition Metals and Coordination Chemistry96 Questions
Exam 20: The Nucleus: a Chemists View73 Questions
Exam 21: Organic and Biochemical Molecules123 Questions
Select questions type
Calculate the solubility of Cu(OH)2 in a solution buffered at pH = 8.50. (Ksp = 1.6 × 10-19)
(Multiple Choice)
4.9/5  (37)
(37)
Calculate the pH of a solution prepared by mixing 50 mL of a 0.10 M solution of HF with 25 mL of a 0.20 M solution of NaF. pKa of HF is 3.14.
(Multiple Choice)
4.8/5  (40)
(40)
Calculate the pH when 200.0 mL of a 1.00 M solution of H2A (Ka1 = 1.0 × 10-6, Ka2 = 1.0 × 10-10) is titrated with the following volumes of 1.00 M NaOH.
-150.0 mL of 1.00 M NaOH
(Multiple Choice)
4.8/5  (38)
(38)
A 200.0-mL sample of the weak acid H3A (0.100 M) is titrated with 0.200 M NaOH. What are the major species at each of the following points in the titration? (Water is always assumed to be a major species.)
-After 200.0 mL of 0.200 M NaOH is added
(Multiple Choice)
4.8/5  (43)
(43)
A solution of hydrochloric acid of unknown concentration was titrated with 0.12 M NaOH. If a 250-mL sample of the HCl solution required exactly 24 mL of the NaOH solution to reach the equivalence point, what was the pH of the HCl solution?
(Multiple Choice)
4.7/5  (37)
(37)
A 75.0-mL sample of 0.0500 M HCN (Ka = 6.2 × 10-10) is titrated with 0.500 M NaOH. What is [H+] in the solution after 3.0 mL of 0.500 M NaOH has been added?
(Multiple Choice)
4.7/5  (29)
(29)
Consider the titration of 100.0 mL of 0.250 M aniline (Kb = 3.8 × 10-10) with 0.500 M HCl. For calculating the volume of HCl required to reach a pH of 8.0, which of the following expressions is correct? (x = volume, in milliliters, of HCl required to reach a pH of 8.0)
(Multiple Choice)
4.8/5  (34)
(34)
Explain why we cannot directly compare Ksp values in comparing solubilities of ionic solids with different numbers of ions.
(Short Answer)
4.9/5  (41)
(41)
What volume of 0.0100 M NaOH must be added to 1.00 L of 0.0500 M HOCl to achieve a pH of 8.00? Ka for HOCl is 3.5 × 10-8.
(Multiple Choice)
4.9/5  (37)
(37)
A 200.0-mL sample of the weak acid H3A (0.100 M) is titrated with 0.200 M NaOH. What are the major species at each of the following points in the titration? (Water is always assumed to be a major species.)
-After 0 mL of 0.200 M NaOH is added
(Multiple Choice)
4.9/5  (31)
(31)
A solution contains 0.50 (Ka = 2.0 × 10-8) and 0.22 M NaA. Calculate the pH after 0.05mol of NaOH is added to 1.00 L of this solution.
(Multiple Choice)
4.9/5  (40)
(40)
The value of Kf for the complex ion Ag(NH3)2+ is 1.7 × 107. Ksp for AgCl is 1.6 × 10-10. Calculate the molar solubility of AgCl in 1.0 M NH3.
(Multiple Choice)
4.8/5  (26)
(26)
Silver chromate, Ag2CrO4, has a Ksp of 9.0 × 10-12. Calculate the solubility, in moles per liter, of silver chromate.
(Multiple Choice)
4.9/5  (34)
(34)
A student titrates an unknown weak acid, HA, to a pale pink phenolphthalein endpoint with 25.0 mL of 0.100 M NaOH. The student then adds 13.0 mL of 0.100 M HCl. The pH of the resulting solution is 4.7. Which of the following statements is true?
(Multiple Choice)
4.8/5  (32)
(32)
Consider the following information about the diprotic acid ascorbic acid (H2As for short, molar mass = 176.1).  The titration curve for disodium ascorbate, Na2As, with standard HCl is shown below:
The titration curve for disodium ascorbate, Na2As, with standard HCl is shown below: 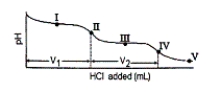 -What is the pH at point III?
-What is the pH at point III?
(Multiple Choice)
4.7/5  (33)
(33)
The Ksp value for PbSO4(s) is 1.3 × 10-8. Calculate the solubility, in moles per liter, of PbSO4(s) in a 0.0010 M solution of Na2SO4.
(Multiple Choice)
4.9/5  (37)
(37)
The Ksp of Al(OH)3 is 2.0 × 10-32. At what pH will a 0.2 M Al3+ solution begin to show precipitation of Al(OH)3?
(Multiple Choice)
4.8/5  (36)
(36)
Consider a 100.0-mL sample of a 0.10 M H2A (Ka1 = 1.3 × 10-3 , Ka2 = 3.1 × 10-6) with 0.10 M NaOH. Determine the pH when 0 mL, 50.0 mL, 100.0 mL, and 150.0 mL of 0.10 M NaOH are added.
(Essay)
4.7/5  (40)
(40)
Calculate the solubility of Ag2SO4 [Ksp = 1.2 × 10-5] in a 2.0 M AgNO3 solution.
(Multiple Choice)
4.8/5  (44)
(44)
A solution contains 10. mmol of H3PO4 and 5.0 mmol of NaH2PO4. How many milliliters of 0.10 M NaOH must be added to reach the second equivalence point of the titration of the H3PO4 with NaOH?
(Multiple Choice)
4.9/5  (40)
(40)
Showing 121 - 140 of 177
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)