Exam 19: The Kinetic Theory of Gases
Exam 1: Measurement37 Questions
Exam 2: Motion Along a Straight Line90 Questions
Exam 3: Vector37 Questions
Exam 4: Motion in Two and Three Dimensions56 Questions
Exam 5: Force and Motion I73 Questions
Exam 6: Force and Motion II74 Questions
Exam 7: Kinetic Energy and Work73 Questions
Exam 8: Potential Energy and Conservation of Energy63 Questions
Exam 9: Center of Mass and Linear Momentum99 Questions
Exam 10: Rotation102 Questions
Exam 11: Rolling, Torque, and Angular Momentum66 Questions
Exam 12: Equilibrium and Elasticity57 Questions
Exam 13: Gravitation55 Questions
Exam 14: Fluids88 Questions
Exam 15: Oscillations75 Questions
Exam 16: Waves I82 Questions
Exam 17: Waves II71 Questions
Exam 18: Temperature, Heat, and the First Law of Thermodynamics96 Questions
Exam 19: The Kinetic Theory of Gases113 Questions
Exam 20: Entropy and the Second Law of Thermodynamics61 Questions
Exam 21: Electric Charge52 Questions
Exam 22: Electric Fields55 Questions
Exam 23: Gauss Law38 Questions
Exam 24: Electric Potential52 Questions
Exam 25: Capacitance61 Questions
Exam 26: Current and Resistance55 Questions
Exam 27: Circuits73 Questions
Exam 28: Magnetic Fields55 Questions
Exam 29: Magnetic Fields Due to Currents49 Questions
Exam 30: Induction and Inductance90 Questions
Exam 31: Electromagnetic Oscillations and Alternating Current88 Questions
Exam 32: Maxwells Equations; Magnetism of Matter81 Questions
Exam 33: Electromagnetic Waves83 Questions
Exam 34: Images79 Questions
Exam 35: Interference46 Questions
Exam 36: Diffraction77 Questions
Exam 37: Relativity68 Questions
Exam 38: Photons and Matter Waves57 Questions
Exam 39: More About Matter Waves41 Questions
Exam 40: All About Atoms79 Questions
Exam 41: Conduction of Electricity in Solids51 Questions
Exam 42: Nuclear Physics68 Questions
Exam 43: Energy From the Nucleus50 Questions
Exam 44: Quarks, Leptons, and the Big Bang55 Questions
Select questions type
An air bubble doubles in volume as it rises from the bottom of a lake (1000 kg/m3). Ignoring any temperature changes, the depth of the lake is:
(Multiple Choice)
4.7/5  (32)
(32)
The temperature of low pressure hydrogen is reduced from 100 C to 20 C. The rms speed of its molecules decreases by approximately:
(Multiple Choice)
4.7/5  (34)
(34)
Five molecules have speeds of 2.8, 3.2, 5.8, 7.3, and 7.4 m/s. Their root-mean-square speed is closest to:
(Multiple Choice)
4.8/5  (30)
(30)
The diagram shows three isotherms for an ideal gas, with T3-T2 the same as T2-T1. It also shows five thermodynamic processes carried out on the gas. Rank the processes in order of the change in the internal energy of the gas, least to greatest. 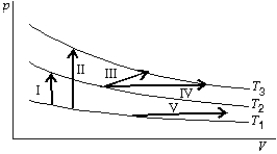
(Multiple Choice)
4.7/5  (33)
(33)
According to the Maxwellian speed distribution, as the temperature increases the number of molecules with speeds within a small interval near the most probable speed:
(Multiple Choice)
4.8/5  (36)
(36)
According to the kinetic theory of gases, the pressure of a gas is due to:
(Multiple Choice)
4.8/5  (46)
(46)
TV is constant for an ideal gas undergoing an adiabatic process, where is the ratio of heat capacities Cp/Cv. This is a direct consequence of:
(Multiple Choice)
4.7/5  (32)
(32)
The average speed of air molecules at room temperature is about:
(Multiple Choice)
4.9/5  (38)
(38)
In order that a single process be both isothermal and occur at constant pressure:
(Multiple Choice)
4.8/5  (43)
(43)
According to the Maxwellian speed distribution, as the temperature increases the average speed:
(Multiple Choice)
4.8/5  (37)
(37)
An ideal gas of N monatomic molecules is in thermal equilibrium with an ideal gas of the same number of diatomic molecules and equilibrium is maintained as temperature is increased. The ratio of the changes in the internal energies ?Edia / ?Emon is:
(Multiple Choice)
4.9/5  (35)
(35)
A gas is confined to a cylindrical container of radius 1 cm and length 1 m. The pressure exerted on an end face, compared with the pressure exerted on the long curved face, is:
(Multiple Choice)
5.0/5  (38)
(38)
Assume that helium behaves as an ideal monatomic gas. If 2 moles of helium undergo a temperature increase of 100 K at constant volume, how much work is done by the gas?
(Multiple Choice)
4.9/5  (35)
(35)
An automobile tire is pumped up to a gauge pressure of 2.0 * 105 Pa when the temperature is 27 C. What is its gauge pressure after the car has been running on a hot day so that the tire temperature is 77 C? Assume that the volume remains fixed and take atmospheric pressure to be 1.013 *105 Pa.
(Multiple Choice)
4.8/5  (42)
(42)
Monatomic, diatomic, and polyatomic ideal gases each undergo slow adiabatic expansions from the same initial volume and the same initial pressure to the same final volume. The magnitude of the work done by the environment on the gas:
(Multiple Choice)
4.8/5  (20)
(20)
The energy absorbed as heat by an ideal gas for an isothermal process equals:
(Multiple Choice)
4.9/5  (35)
(35)
The specific heat at constant volume of an ideal gas depends on:
(Multiple Choice)
4.8/5  (43)
(43)
Showing 21 - 40 of 113
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)