Exam 16: Aromatic Compounds
Exam 1: Introduction and Review127 Questions
Exam 2: Structure and Properties of Organic Molecules129 Questions
Exam 3: Structure and Stereochemistry of Alkanes129 Questions
Exam 4: The Study of Chemical Reactions128 Questions
Exam 5: Stereochemistry128 Questions
Exam 6: Alkyl Halides: Nucleophilic Substitution and Elimination132 Questions
Exam 7: Structure and Synthesis of Alkenes128 Questions
Exam 8: Reactions of Alkenes132 Questions
Exam 9: Alkynes124 Questions
Exam 10: Structure and Synthesis of Alcohols132 Questions
Exam 11: Reactions of Alcohols124 Questions
Exam 12: Infrared Spectroscopy and Mass Spectrometry120 Questions
Exam 13: Nuclear Magnetic Resonance Spectroscopy130 Questions
Exam 14: Ethers, Epoxides, and Thioethers127 Questions
Exam 15: Conjugated Systems, Orbital Symmetry, and Ultraviolet Spectroscopy130 Questions
Exam 16: Aromatic Compounds128 Questions
Exam 17: Reactions of Aromatic Compounds129 Questions
Exam 18: Ketones and Aldehydes132 Questions
Exam 19: Amines129 Questions
Exam 20: Carboxylic Acids125 Questions
Exam 21: Carboxylic Acid Derivatives128 Questions
Exam 22: Condensations and Alpha Substitutions of Carboxyl Compounds125 Questions
Exam 23: Carbohydrates and Nucleic Acids125 Questions
Exam 24: Amino Acids, Peptides, and Proteins127 Questions
Exam 25: Lipids127 Questions
Exam 26: Synthetic Polymers128 Questions
Select questions type
Classify the compound below as aromatic antiaromatic, or nonaromatic. Assume planarity of the π network. 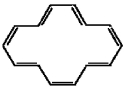
(Short Answer)
4.8/5  (49)
(49)
Which of the following compounds has the lowest boiling point?
(Multiple Choice)
4.7/5  (35)
(35)
Classify cycloheptatrienyl cation as aromatic, antiaromatic, or nonaromatic. Assume planarity of the π network.
(Short Answer)
4.8/5  (37)
(37)
How might one distinguish the isomers of trimethylbenzene by noise-decoupled 13C NMR?
(Essay)
4.9/5  (29)
(29)
Add six pi bonds to the structure below to produce the most stable isomer. 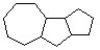
(Essay)
4.8/5  (30)
(30)
Why are researchers interested in the properties of large polynuclear aromatic hydrocarbons?
(Essay)
4.8/5  (32)
(32)
Classify the following compound as being aromatic, nonaromatic or antiaromatic. 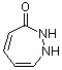
(Short Answer)
4.8/5  (37)
(37)
Which of the following is an incorrect description of benzene?
(Multiple Choice)
4.8/5  (36)
(36)
How many pairs of degenerate π molecular orbitals are found in benzene?
(Multiple Choice)
4.8/5  (41)
(41)
Which of the following is also an acceptable name for 3-nitrophenol?
(Multiple Choice)
4.9/5  (39)
(39)
Classify the compound below as aromatic, antiaromatic, or nonaromatic. Assume planarity of the π network. 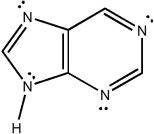
(Short Answer)
4.8/5  (42)
(42)
Classify the compound below as aromatic, antiaromatic, or nonaromatic. Assume planarity of the π network. 
(Short Answer)
4.7/5  (42)
(42)
Nitrogen's lone pair electrons occupy what type of orbital in pyridine?
(Multiple Choice)
4.7/5  (30)
(30)
What is suggested by the fact that benzene's molar heat of hydrogenation is 36 kcal less than three times the molar heat of hydrogenation of cyclohexene?
(Essay)
4.8/5  (43)
(43)
Classify the compound below as aromatic, antiaromatic, or nonaromatic. Assume planarity of the π network. 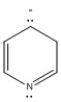
(Short Answer)
4.8/5  (39)
(39)
Classify pyrrole as aromatic, antiaromatic, or nonaromatic. Assume planarity of the π network.
(Short Answer)
5.0/5  (40)
(40)
Showing 101 - 120 of 128
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)