Multiple Choice
Hidden Assumptions that make Equilibrium Calculations Easier What Do We Do When the Approximation Fails?
based on the following reaction:
2H2O(g) 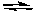 2H2(g) + O2(g)
2H2(g) + O2(g)
Kc = 2 x 10-42 (at 25oC)
-When solving a problem with the initial conditions outlined below, we rearrange the problem to give an intermediate set of conditions where the concentration of one of the reactants or products is equal to zero.
Fe(CO) 5 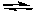 Fe(CO) 4 + CO
Fe(CO) 4 + CO
Initial: 0.10 M 0.10 M 0.005 M
Our choice of whether to push the reaction all the way to the right or all the way to the left is determined by which of the following goals?
A) to make both Qc and Kc large
B) to make both Qc and Kc small
C) to bring Qc as close as possible to Kc
D) to make the difference between Qc and Kc as large as possible
Correct Answer:

Verified
Correct Answer:
Verified
Q33: For the reaction<br>2 NOCl(g) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB9692/.jpg" alt="For
Q34: The equilibrium constant for the following reaction
Q35: If at any moment in time, we
Q36: Which of the following equations correctly describes
Q37: What would happen to the extent of
Q39: Hidden Assumptions that make Equilibrium Calculations
Q40: Hidden Assumptions that make Equilibrium Calculations Easier
Q41: The addition of I<sub>2</sub>(g) to a fixed
Q42: A solution is prepared in which the
Q43: Which of the following equations correctly describes