Exam 7: Enzyme Mechanisms
Exam 1: Principles of Biochemistry100 Questions
Exam 2: Physical Biochemistry: Energy Conversion, Water, and Membranes100 Questions
Exam 3: Nucleic Acid Structure and Function100 Questions
Exam 4: Protein Structure100 Questions
Exam 5: Methods in Protein Biochemistry100 Questions
Exam 6: Protein Function114 Questions
Exam 7: Enzyme Mechanisms106 Questions
Exam 8: Cell Signaling Systems102 Questions
Exam 9: Glycolysis: a Paradigm of Metabolic Regulation100 Questions
Exam 10: The Citrate Cycle100 Questions
Exam 11: Oxidative Phosphorylation99 Questions
Exam 12: Photosynthesis100 Questions
Exam 13: Carbohydrate Structure and Function100 Questions
Exam 14: Carbohydrate Metabolism100 Questions
Exam 15: Lipid Structure and Function100 Questions
Exam 16: Lipid Metabolism100 Questions
Exam 17: Amino Acid Metabolism100 Questions
Exam 18: Nucleotide Metabolism99 Questions
Exam 19: Metabolic Integration101 Questions
Exam 20: DNA Replication, Repair, and Recombination99 Questions
Exam 21: RNA Synthesis, Processing, and Gene Silencing100 Questions
Exam 22: Protein Synthesis, Posttranslational Modification, and Transport100 Questions
Exam 23: Gene Regulation100 Questions
Select questions type
In vivo arginine decarboxylase activity is monitored over several days in the absence and presence of protease inhibitors. The activity is greatly reduced when protease inhibitors are present. Arginine decarboxylase is not a protease. Interpret these findings.
Free
(Essay)
4.9/5  (32)
(32)
Correct Answer:
Arginine decarboxylase activity is regulated by proteolytic processing. When the proteases responsible for activating arginine decarboxylase are inhibited, the activity of arginine decarboxylase is reduced.
If the rate constant for a reaction is determined to be equal to v / [Q][R], the reaction is the conversion of
Free
(Multiple Choice)
4.8/5  (33)
(33)
Correct Answer:
C
The conversion of L-proline to D-proline is shown below. Which of the following characteristics would most likely be found in a transition state analog that inhibits an enzyme that catalyzes the reaction? 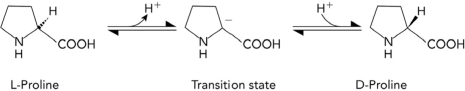
(Multiple Choice)
4.9/5  (33)
(33)
Procathepsin B is a lysosomal protease that is first translated as a proenzyme. On autocleavage it is fully activated. Procathepsin B is
(Multiple Choice)
4.8/5  (41)
(41)
Reaction coordinate diagrams clearly show that the energy of an enzyme bound to a transition state is higher than the energies of the E + S, E + P, and ES that occur along the same reaction coordinate. The energy of an enzyme bound to a transition state analog would lie in the diagram.
(Multiple Choice)
4.8/5  (30)
(30)
The hemithioacetal intermediate formed during the action of HMG-CoA reductase is stabilized by
(Multiple Choice)
4.9/5  (40)
(40)
Which pair correctly matches the coenzymes most often used to mediate the described redox reactions?
(Multiple Choice)
4.7/5  (36)
(36)
The Lineweaver-Burk plot shows data obtained for an enzyme in the absence and presence of a noncompetitive inhibitor. If the [I] is increased significantly in the experiment, the Vmaxapp would and the K app would _. ![The Lineweaver-Burk plot shows data obtained for an enzyme in the absence and presence of a noncompetitive inhibitor. If the [I] is increased significantly in the experiment, the V<sub>max</sub><sup>app</sup> would and the K <sup>app </sup><sup> </sup>would _.](https://storage.examlex.com/TB1187/11eaca6f_bd0c_1ebd_9abe_fb708d4e2e83_TB1187_00.jpg)
![The Lineweaver-Burk plot shows data obtained for an enzyme in the absence and presence of a noncompetitive inhibitor. If the [I] is increased significantly in the experiment, the V<sub>max</sub><sup>app</sup> would and the K <sup>app </sup><sup> </sup>would _.](https://storage.examlex.com/TB1187/11eaca6f_bd0c_1ebe_9abe_ef770293c81c_TB1187_00.jpg)
(Multiple Choice)
4.9/5  (32)
(32)
Consider the reaction coordinate diagram shown below for the conversion of substrate (S) to product (P) via two different reaction pathways that involve different transition states
(‡ and ‡*). Assume that one pathway involves a catalyst and one does not. Decide which pathway involves the catalyst and explain your logic.
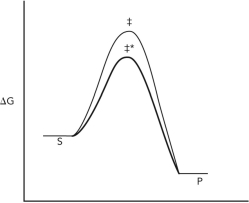
(Essay)
4.8/5  (36)
(36)
The glutamate side chain in the active site of HMG-CoA reductase acts as a general base only after
(Multiple Choice)
4.7/5  (32)
(32)
Enzymes from four different species catalyze the same reaction. Based on the reaction coordinate diagrams below, which species contains an enzyme that experiences more bonding interactions with the transition state of the reaction? 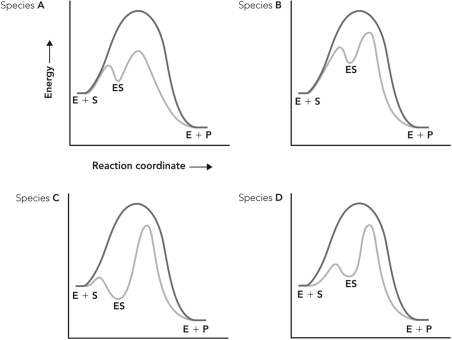
(Multiple Choice)
4.9/5  (39)
(39)
The activity of an enzyme is monitored as a function of temperature. At low temperature very little activity is seen; the activity peaks as the temperature increases and then dramatically decreases to zero as higher temperatures are attained. Explain why the activity drops off completely at higher temperatures.
(Essay)
4.7/5  (45)
(45)
Which of the following is NOT a function of the Mg2+ ions in the mechanism of enolase?
(Multiple Choice)
4.7/5  (32)
(32)
The three general categories of enzyme-mediated reactions, which are determined on the basis of the work they accomplish, include all EXCEPT
(Multiple Choice)
4.8/5  (37)
(37)
Given the following data for lactate dehydrogenase, calculate the specificity constant. 
(Essay)
4.8/5  (33)
(33)
Showing 1 - 20 of 106
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)