Exam 14: Energy Generation in Mitochondria and Chloroplasts
Exam 1: Cells: The Fundamental Units of Life64 Questions
Exam 2: Chemical Components of Cells74 Questions
Exam 3: Energy, Catalysis, and Biosynthesis73 Questions
Exam 4: Protein Structure and Function71 Questions
Exam 5: DNA and Chromosomes69 Questions
Exam 6: DNA Replication and Repair61 Questions
Exam 7: From DNA to Protein62 Questions
Exam 8: Control of Gene Expression68 Questions
Exam 9: How Genes and Genomes Evolve60 Questions
Exam 10: Analyzing the Structure and Function of Genes59 Questions
Exam 11: Membrane Structure57 Questions
Exam 12: Transport Across Cell Membranes67 Questions
Exam 13: How Cells Obtain Energy From Food71 Questions
Exam 14: Energy Generation in Mitochondria and Chloroplasts72 Questions
Exam 15: Intracellular Compartments and Protein Transport55 Questions
Exam 16: Cell Signaling60 Questions
Exam 17: Cytoskeleton59 Questions
Exam 18: The Cell-Division Cycle67 Questions
Exam 19: Sexual Reproduction and the Power of Genetics61 Questions
Exam 20: Cell Communities: Tissues, Stem Cells, and Cancer57 Questions
Select questions type
Describe how a standard flashlight battery can convert energy into useful work and explain how this is similar to the energy conversions in the mitochondria.
Free
(Essay)
4.8/5  (42)
(42)
Correct Answer:
A battery contains chemicals that generate negatively charged ions at one pole, and it is able to cause the continuous transfer of electrons along a metal wire if that pole is connected to the other end of the battery.The energy released by the electron-transfer process driven by the battery can be harnessed to do useful work, as when it is used to run an electric motor.Likewise, the energy released by the electron transfers that occur between the protein complexes in the electron-transport chain does useful work when it drives the movement of protons to one side of the membrane, since the resulting proton gradient is then used to generate chemical energy in the form of ATP.
Which of the following statements about the possible fates of glyceraldehyde 3-phosphate is FALSE?
Free
(Multiple Choice)
4.8/5  (35)
(35)
Correct Answer:
D
Which of the phylogenetic trees in Figure 14-47 is the most accurate? Explain your answer.Note: the mitochondria and chloroplasts are from maize, but they are treated as independent "organisms" for the purposes of this question. (a)
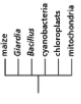 (c)
(c)
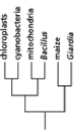 (e)
(e)
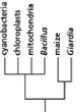 (b)
(b)
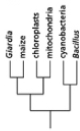 (d)
(d)
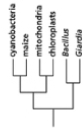 Figure 14-47
Figure 14-47
Free
(Multiple Choice)
4.9/5  (39)
(39)
Correct Answer:
C
Which ratio of NADH to NAD+ in solution will generate the largest positive redox potential?
(Multiple Choice)
4.9/5  (35)
(35)
For each of the following sentences, choose one of the options enclosed in square brackets to make a correct statement.
An electron bound to a molecule with low affinity for electrons is a [high/low]-energy electron.Transfer of an electron from a molecule with low affinity to one with higher affinity has a [positive/negative] ΔG° and is thus [favorable/unfavorable] under standard conditions.If the reduced form of a redox pair is a strong electron donor with a [high/low] affinity for electrons, it is easily oxidized; the oxidized member of such a redox pair is a [weak/strong] electron acceptor.
(Essay)
4.9/5  (29)
(29)
Indicate whether the following statements are TRUE or FALSE.If a statement is false, explain why it is false.
Correct Answer:
Premises:
Responses:
(Matching)
4.7/5  (37)
(37)
The overall relationship that links bond-forming reactions to membrane transport processes in the mitochondria is called
(Multiple Choice)
5.0/5  (38)
(38)
In the electron-transport chain in chloroplasts, __________-energy electrons are taken from __________.
(Multiple Choice)
4.8/5  (39)
(39)
Stage 2 of photosynthesis, sometimes referred to as the dark reactions, involves the reduction of CO2 to produce organic compounds such as sucrose.What cofactor is the electron donor for carbon fixation?
(Multiple Choice)
4.8/5  (39)
(39)
Which of the following statements about "redox potential" is TRUE?
(Multiple Choice)
4.7/5  (38)
(38)
Which component of the electron-transport chain is required to combine the pair of electrons with molecular oxygen?
(Multiple Choice)
4.9/5  (28)
(28)
Mitochondrial structure and the reaction products generated inside the matrix are critical for generating stores of energy.Answer the following questions based on what you know about mitochondrial structure and processes.
A.The gradients used to generate ATP are maintained across the inner mitochondrial membrane.Why don't we observe a similar gradient generation across the outer mitochondrial membrane?
B.The proton-motive force created by the electrochemical proton gradient is the source of free energy utilized in ATP formation.Describe the two components contributing to the total proton-motive force.
(Essay)
4.8/5  (32)
(32)
Below is a list of breakthroughs in energy metabolism in living systems.Which is the correct order in which they are thought to have evolved?
A)H2O-splitting enzyme activity
B)light-dependent transfer of electrons from H2S to NADPH
C)the consumption of fermentable organic acids
D)oxygen-dependent ATP synthesis
A)A, C, D, B
B)C, A, B, D
C)B, C, A, D
D)C, B, A, D
(Short Answer)
4.8/5  (38)
(38)
The citric acid cycle generates NADH and FADH2, which are then used in the process of oxidative phosphorylation to make ATP.The reactions in the citric acid cycle do not utilize oxygen.Yet the citric acid cycle stops almost immediately when O2 is removed.Explain this observation.
(Essay)
4.8/5  (28)
(28)
Indicate whether the following statements are TRUE or FALSE.If a statement is false, explain why it is false.
Correct Answer:
Premises:
Responses:
(Matching)
4.7/5  (34)
(34)
The relationship of free-energy change (ΔG) to the concentrations of reactants and products is important because it predicts the direction of spontaneous chemical reactions.Consider, for example, the hydrolysis of ATP to ADP and inorganic phosphate (Pi).The standard free-energy change (ΔG°) for this reaction is −7.3 kcal/mole.The free-energy change depends on concentrations according to the following equation:
ΔG = ΔG° + 1.42 log10 ([ADP] [Pi]/[ATP])
In a resting muscle, the concentrations of ATP, ADP, and Pi are approximately 0.005 M, 0.001 M, and 0.010 M, respectively.At [Pi] = 0.010 M, what will be the ratio of [ATP] to [ADP] at equilibrium?
(Multiple Choice)
4.9/5  (34)
(34)
Some bacteria can live both aerobically and anaerobically.How does the ATP synthase in the plasma membrane of the bacterium help such bacteria to keep functioning in the absence of oxygen?
(Essay)
4.8/5  (36)
(36)
Which of the following statements describes the phosphorylation event that occurs during the process known as "oxidative phosphorylation"?
(Multiple Choice)
4.9/5  (34)
(34)
The relationship of free-energy change (ΔG) to the concentrations of reactants and products is important because it predicts the direction of spontaneous chemical reactions.In the hydrolysis of ATP to ADP and inorganic phosphate (Pi), the standard free-energy change (ΔG°) is −7.3 kcal/mole.The free-energy change depends on concentrations according to the following equation:
ΔG = ΔG° + 1.42 log10 ([ADP] [Pi]/[ATP])
In a resting muscle, the concentrations of ATP, ADP, and Pi are approximately 0.005 M, 0.001 M, and 0.010 M, respectively.What is the ΔG for ATP synthesis in resting muscle?
(Multiple Choice)
4.8/5  (41)
(41)
Showing 1 - 20 of 72
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)