Exam 3: Energy, Catalysis, and Biosynthesis
Exam 1: Cells: The Fundamental Units of Life64 Questions
Exam 2: Chemical Components of Cells74 Questions
Exam 3: Energy, Catalysis, and Biosynthesis73 Questions
Exam 4: Protein Structure and Function71 Questions
Exam 5: DNA and Chromosomes69 Questions
Exam 6: DNA Replication and Repair61 Questions
Exam 7: From DNA to Protein62 Questions
Exam 8: Control of Gene Expression68 Questions
Exam 9: How Genes and Genomes Evolve60 Questions
Exam 10: Analyzing the Structure and Function of Genes59 Questions
Exam 11: Membrane Structure57 Questions
Exam 12: Transport Across Cell Membranes67 Questions
Exam 13: How Cells Obtain Energy From Food71 Questions
Exam 14: Energy Generation in Mitochondria and Chloroplasts72 Questions
Exam 15: Intracellular Compartments and Protein Transport55 Questions
Exam 16: Cell Signaling60 Questions
Exam 17: Cytoskeleton59 Questions
Exam 18: The Cell-Division Cycle67 Questions
Exam 19: Sexual Reproduction and the Power of Genetics61 Questions
Exam 20: Cell Communities: Tissues, Stem Cells, and Cancer57 Questions
Select questions type
When there is an excess of nutrients available in the human body, insulin is released to stimulate the synthesis of glycogen from glucose.This is a specific example of a/an __________ process, a general process in which larger molecules are made from smaller molecules.
Free
(Multiple Choice)
4.8/5  (32)
(32)
Correct Answer:
C
A chemical reaction is defined as spontaneous if there is a net loss of free energy during the reaction process.However, spontaneous reactions do not always occur rapidly.Favorable biological reactions require __________ to selectively speed up reactions and meet the demands of the cell.
Free
(Multiple Choice)
4.8/5  (24)
(24)
Correct Answer:
D
Consider a description of an enzymatic reaction pathway that begins with the binding of substrate S to enzyme E, and ends with the release of product P from the enzyme.
E + S → ES → EP → E + P
In many circumstances,
Km = [E][S]/[ES]
A.What proportion of enzyme molecules is bound to substrate when [S] = Km?
B.Recall that when [S] = Km, the reaction rate is ½Vmax.Does your answer to part A make sense in the light of this rate information?
Free
(Essay)
4.9/5  (37)
(37)
Correct Answer:
A.When [S] is substituted for Km in the equation, it becomes clear that [E] = [ES].Thus, half of the enzyme molecules are free and half are bound to the substrate.
B.Yes.If half of the enzyme molecules are bound to the substrate, it makes intuitive sense that the reaction rate is half of the maximum possible rate, or half of the rate observed when all of the enzyme molecules are bound to the substrate.
A.You are measuring the effect of temperature on the rate of an enzyme-catalyzed reaction.If you plot reaction rate against temperature, which of the graphs in Figure 3-64 would you expect your plot to resemble?
B.Explain why temperature has this effect. 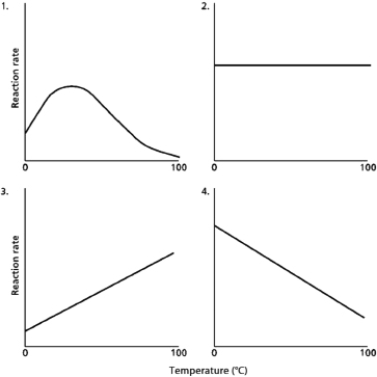 Figure 3-64
Figure 3-64
(Essay)
4.9/5  (43)
(43)
Hydrolysis reactions are commonly used inside the cell to split high-energy covalent bonds.For each of the three reactions below, use the ΔG° for each reaction to determine the equilibrium constants (K).Assume that each reaction occurs independently of the other two.
ΔG° (kcal/mole)
Reaction 1: acetyl-P → acetate + P −10.3
Reaction 2: ATP → ADP + P −7.3
Reaction 3: glucose 6-P → glucose + P −3.3
(Essay)
4.8/5  (34)
(34)
If you weigh yourself on a scale one morning, then eat four pounds of food during the day, will you weigh four pounds more the next morning? Why or why not? (Hint: What happens to the atoms from the food you ingested?)
(Essay)
4.8/5  (32)
(32)
ΔG° indicates the change in the standard free energy as a reactant is converted to product.Given what you know about these values, which reaction below is the most favorable?
(Multiple Choice)
4.9/5  (31)
(31)
Oxidation is a favorable process in an aerobic environment, which is the reason cells are able to derive energy from the oxidation of macromolecules.Once carbon has been oxidized to __________, its most stable form, it can only cycle back into the organic portion of the carbon cycle through __________.
(Multiple Choice)
4.9/5  (32)
(32)
The addition of a new deoxynucleotide to a growing DNA chain requires more energy than can be obtained by the hydrolysis of ATP to ADP + Pi.What alternative series of reactions is used, and how does this help overcome the energy barrier for DNA synthesis?
(Essay)
4.8/5  (37)
(37)
ΔG measures the change of free energy in a system as it converts reactant (Y) into product (X).When [Y] = [X], ΔG is equal to
(Multiple Choice)
4.8/5  (32)
(32)
Even though cellular macromolecules contain a large number of carbon and hydrogen atoms, they are not all spontaneously converted into CO2 and H2O.This absence of spontaneous combustion is due to the fact that biological molecules are relatively __________ and an input of energy is required to reach lower energy states.
(Multiple Choice)
4.8/5  (31)
(31)
Isomerization of glucose 1-phosphate to glucose 6-phosphate is energetically favorable.At 37°C, ΔG° = −1.42 log10K.What is the equilibrium constant for this reaction if ΔG° = −1.74 kcal/mole at 37°C?
(Multiple Choice)
4.9/5  (28)
(28)
Which of the following statements is FALSE for a favorable binding reaction?
(Multiple Choice)
4.7/5  (34)
(34)
Oxidation is the process by which oxygen atoms are added to a target molecule.Generally, the atom that is oxidized will experience which of the following with respect to the electrons in its outer shell?
(Multiple Choice)
4.9/5  (37)
(37)
The equilibrium constant for complex formation between molecules A and B will depend on their relative concentrations, as well as the rates at which the molecules associate and dissociate.The association rate will be larger than the dissociation rate when complex formation is favorable.The energy that drives this process is referred to as __________ energy.
(Multiple Choice)
4.8/5  (32)
(32)
For each of the pairs A-D in Figure 3-54, pick the more reduced member of the pair. (i) (ii)
(A)
(B)
(C)
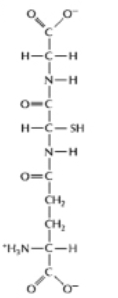
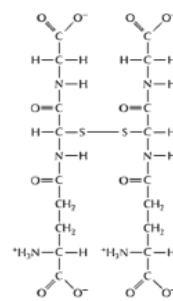 (D)
Figure 3-54
(D)
Figure 3-54
(Essay)
4.9/5  (39)
(39)
In general, there is a positive change in free energy associated with reduction reactions, and most of them are coupled with oxidation reactions.The last step in the biosynthesis of cholesterol involves the reduction of a carbon-carbon double bond.What activated carrier molecule is used in this reaction (and generally for the reduction of lipids) and how would this reaction be influenced by the levels of available ATP?
(Essay)
4.8/5  (45)
(45)
The graph in Figure 3-34 illustrates the change in the rate of an enzyme-catalyzed reaction as the concentration of substrate is increased.Which of the values listed below is used to calculate the enzyme turnover number? 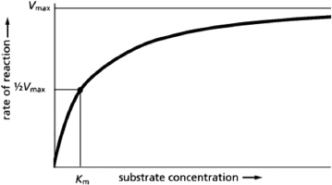 Figure 3-34
Figure 3-34
(Multiple Choice)
4.8/5  (33)
(33)
The anhydride formed between a carboxylic acid and a phosphate (Figure 3-45A) is a high-energy intermediate for some reactions in which ATP is the energy source.Arsenate can also be incorporated into a similar high-energy intermediate in place of the phosphate (Figure 3-45B).Figure 3-45C shows the reaction profiles for the hydrolysis of these two high-energy intermediates.What is the effect of substituting arsenate for phosphate in this reaction? (A)
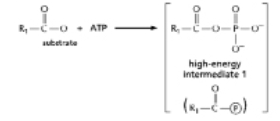 (B)
(B)
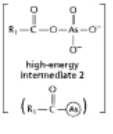
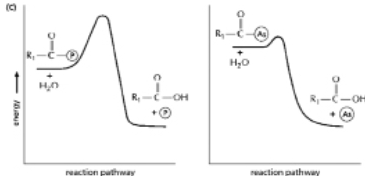 Figure 3-45
Figure 3-45
(Multiple Choice)
4.9/5  (29)
(29)
Your body extracts energy from the food you ingest by catalyzing reactions that essentially "burn" the food molecules in a stepwise fashion.What is another way to describe this process?
(Multiple Choice)
4.7/5  (38)
(38)
Showing 1 - 20 of 73
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)