Exam 4: Protein Structure and Function
Proteins bind selectively to small-molecule targets called ligands.The selection of one ligand out of a mixture of possible ligands depends on the number of weak, noncovalent interactions in the protein's ligand-binding site.Where is the binding site typically located in the protein structure?
B
You wish to produce a human enzyme, protein A, by introducing its gene into bacteria.The genetically engineered bacteria make large amounts of protein A, but it is in the form of an insoluble aggregate with no enzymatic activity.Which of the following procedures might help you to obtain soluble, enzymatically active protein? Select all options that may be useful.Explain your reasoning.
A.Make the bacteria synthesize protein A in smaller amounts.
B.Dissolve the protein aggregate in urea, then dilute the solution and gradually remove the urea.
C.Treat the insoluble aggregate with a protease.
D.Make the bacteria overproduce chaperone proteins in addition to protein A.
E.Heat the protein aggregate to denature all proteins, then cool the mixture.
Choices A, B, and D are all worth trying.Some proteins require molecular chaperones if they are to fold properly within the environment of the cell.In the absence of chaperones, a partly folded polypeptide chain has exposed amino acids that can form noncovalent bonds with other regions of the protein itself and with other proteins, thus causing nonspecific aggregation of proteins.
A.Because the protein you are expressing in bacteria is being made in large quantities, it is possible that there are not enough chaperone molecules in the bacterium to fold the protein.Expressing the protein at lower levels might increase the amount of properly folded protein.
B.Urea should solubilize the protein and completely unfold it.Removing the urea slowly and gradually often allows the protein to refold.Presumably, under less crowded conditions, the protein should be able to refold into its proper conformation.
C.Treating the aggregate with a protease, which cleaves peptide bonds, will probably solubilize the protein by trimming it into pieces that do not interact as strongly with one another; however, chopping up the protein will also destroy its enzymatic activity.
D.Overexpressing chaperone proteins might increase the amount of properly folded protein.
E.Heating can lead to the partial denaturation and aggregation of proteins to form a solid gelatinous mass, as when cooking an egg white, and rarely helps solubilize proteins.
Polypeptides are synthesized from amino acid building blocks.The condensation reaction between the growing polypeptide chain and the next amino acid to be added involves the loss of
A
Motor proteins use the energy in ATP to transport organelles, rearrange elements of the cytoskeleton during cell migration, and move chromosomes during cell division.Which of the following mechanisms is sufficient to ensure the unidirectional movement of a motor protein along its substrate?
The protein structure in Figure 4-76 contains four α helices arranged in a bundle.Label each helix by number (1 to 4) starting from the N-terminus and going to the C-terminus, both of which are labeled.List the six possible pairings of these helices, and indicate within each pair whether the helices are parallel or antiparallel.  Figure 4-76
Figure 4-76
Some proteins have α helices, some have β sheets, and still others have a combination of both.What makes it possible for proteins to have these common structural elements?
Energy required by the cell is generated in the form of ATP.ATP is hydrolyzed to power many of the cellular processes, increasing the pool of ADP.As the relative amount of ADP molecules increases, they can bind to glycolytic enzymes, which will lead to the production of more ATP.The best way to describe this mechanism of regulation is
Protein structures have several different levels of organization.The primary structure of a protein is its amino acid sequence.The secondary and tertiary structures are more complicated.Consider the definitions below and select the one that best fits the term "protein domain."
β sheets can participate in the formation of amyloid fibers, which are insoluble protein aggregates.What drives the formation of amyloid fibers?
Studies conducted with a lysozyme mutant that contains an Asp→Asn change at position 52 and a Glu→Gln change at position 35 exhibited almost a complete loss in enzymatic activity.What is the most likely explanation for the decrease in enzyme activity in the mutant?
You have produced a monoclonal antibody that binds to the protein actin.To be sure that the antibody does not cross-react with other proteins, you test your antibody in a western blot assay on whole-cell lysates that have been subjected to electrophoresis under nondenaturing conditions (shown in Figure 4-69A) and denaturing conditions (shown in Figure 4-69B).Does the antibody cross-react with other proteins? If so, does this explain the results in the two western blots? If not, how do you explain the difference observed? 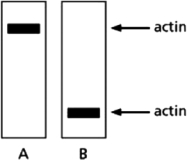 Figure 4-69
Figure 4-69
Molecular chaperones can work by creating an "isolation chamber." What is the purpose of this chamber?
Fill in the blank spaces in the table below.The first row has been completed for you. 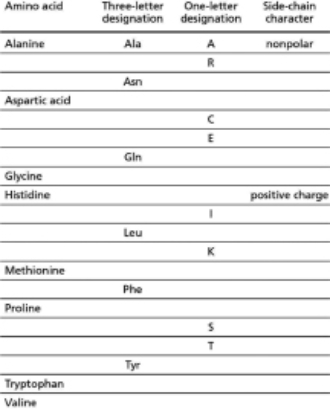 Table 4-75A
Table 4-75A
The three-dimensional coordinates of atoms within a folded protein are determined experimentally.After researchers obtain a protein's structural details, they can use different techniques to highlight particular aspects of the structure.What visual model best displays a protein's secondary structures (α helices and β sheets)?
Protein folding can be studied using a solution of purified protein and a denaturant (urea), a solvent that interferes with noncovalent interactions.Which of the following is observed after the denaturant is removed from the protein solution?
For each of the following, indicate whether the individual folded polypeptide chain forms a globular (G) or fibrous (F) protein molecule.
A.keratin
B.lysozyme
C.elastin
D.collagen
E.hemoglobin
F.actin
Which of the following methods would be the most suitable to assess whether your protein exists as a monomer or in a complex?
Which of the following methods used to study proteins is limited to proteins with a molecular mass of 50 kD or less?
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)