Exam 18: Thermodynamics: Spontaneous and Nonspontaneous Reactions and Processes
Exam 1: Matter and Energy: The Origin of the Universe99 Questions
Exam 2: Atoms, Ions, and Molecules: Matter Starts Here131 Questions
Exam 3: Stoichiometry: Mass, Formulas, and Reactions133 Questions
Exam 4: Solution Chemistry: The Hydrosphere126 Questions
Exam 5: Thermochemistry: Energy Changes in Reactions132 Questions
Exam 6: Properties of Gases: the Air We Breathe138 Questions
Exam 7: A Quantum Model of Atoms: Waves and Particles143 Questions
Exam 8: Chemical Bonds: What Makes a Gas a Greenhouse Gas139 Questions
Exam 9: Molecular Geometry: Shape Determines Function136 Questions
Exam 10: Intermolecular Forces: The Uniqueness of Water140 Questions
Exam 11: Solutions: Properties and Behavior130 Questions
Exam 12: Solids: Structures and Applications144 Questions
Exam 13: Organic Chemistry: Fuels, Pharmaceuticals, Materials, and Life129 Questions
Exam 14: Chemical Kinetics: Reactions in the Air We Breathe164 Questions
Exam 15: Chemical Equilibrium: How Much Product Does a Reaction Really Make91 Questions
Exam 16: Acid-Base and Solubility Equilibria: Reactions in Soil and Water179 Questions
Exam 17: Metal Ions: Colorful and Essential144 Questions
Exam 18: Thermodynamics: Spontaneous and Nonspontaneous Reactions and Processes157 Questions
Exam 19: Electrochemistry: the Quest for Clean Energy143 Questions
Exam 20: Biochemistry: the Compounds of Life108 Questions
Exam 21: Nuclear Chemistry: Applications to Energy and Medicine144 Questions
Exam 22: Life and the Periodic Table95 Questions
Select questions type
The values of H°rxn and S°rxn are shown for the following equilibrium reaction. What makes the equilibrium constant larger at low temperature?
2NO2(g) N2O4(g) H = -58.02 kJ/mol, S°=-176.5 J/mol .K
(Multiple Choice)
4.7/5  (27)
(27)
The equilibrium vapor pressure for benzene is 94.4 mm Hg. When liquid benzene is in equilibrium with its vapor, we must have ________
(Multiple Choice)
4.9/5  (50)
(50)
Determine the normal melting point of benzoic acid in degrees centigrade given the following data:
H \circ fus
= 18.02 kJ/mol
S \circ fus
= 45.56 J/mol . K
(Short Answer)
4.9/5  (44)
(44)
Dinitrogen tetroxide (N2O4) decomposes to nitrogen dioxide (NO2). If H° = 58.02 kJ/mol and S° = 176.1 J/mol · K, at what temperature are reactants and products in their standard states at equilibrium?
(Multiple Choice)
4.9/5  (37)
(37)
Which of the following must be true for a reaction to proceed to form products?
(Multiple Choice)
4.8/5  (47)
(47)
The heat of fusion for water is 333.6 J/g. What is the entropy change for the universe when 1.00 L of water at 0°C freezes at +5°C? Comment on the sign.
(Essay)
4.7/5  (37)
(37)
The symbol G
(CH4, g) refers to which of the following reactions?
(Multiple Choice)
4.7/5  (25)
(25)
The standard free energy of formation, , of atomic oxygen is 230.1 kJ/mol. Determine the equilibrium constant for the following reaction at standard thermodynamic conditions.
O2(g) 2O(g)
(Multiple Choice)
4.7/5  (44)
(44)
Draw a graph of entropy versus temperature for a typical substance. Be sure to clearly label phases and phase transitions on the graph.
(Essay)
4.8/5  (34)
(34)
A sketch of the free energy versus Q for a hypothetical chemical equilibrium is shown here. The stress to the equilibrium by adding more reactants is shown by which arrow? 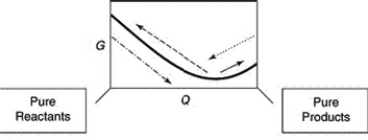
(Multiple Choice)
4.9/5  (38)
(38)
Indicate which one of the following reactions results in a positive Ssys.
(Multiple Choice)
4.9/5  (28)
(28)
In a spontaneous process, the entropy of the universe ________
(Multiple Choice)
4.7/5  (28)
(28)
Which of the following statements about equilibrium are true?
I. Gsys = 0
II. Ssys = 0
III. Suniverse = 0
(Multiple Choice)
4.9/5  (36)
(36)
The standard entropy of nitrogen gas in air is 192 J/(mol . K). Estimate how many states are accessible to a nitrogen molecule in air.
(Multiple Choice)
4.9/5  (36)
(36)
If 3.500 g of Ni are reacted with excess oxygen to form nickel oxide (NiO) under standard state conditions, what is the entropy change for the reaction?
2Ni(s) + O2 2NiO(s) Substance (/\cdot) 182.1 205.0 37.99
(Multiple Choice)
4.9/5  (33)
(33)
A perturbation or stress to a chemical reaction at equilibrium ________
(Multiple Choice)
4.9/5  (38)
(38)
Which statement characterizes the following table?
Temperature Dependence of Reaction Spontaneity \DeltaS<0 \DeltaS>0 \DeltaH<0 Spontaneous only at a sufficiently high temperature. Always spontaneous at any temperature. \DeltaH>0 Never spontaneous at any temperature. Spontaneous only at a sufficiently low temperature.
(Multiple Choice)
4.9/5  (41)
(41)
Alcohols for use as biofuels can be produced from glucose that is obtained from starch and cellulose in plants. Use the information in the table below to determine the temperature range, if any, at which this reaction is spontaneous.
C6H12O6(s) 2CH3CH2OH(l) + 2CO2(g) Compound \Delta [/(\cdot)] Glucose (s) (/) 212 Ethanol (l) -1,274 161 Carbon dioxide (g) -278 214
(Multiple Choice)
4.8/5  (32)
(32)
The entropy of vaporization of water is 109.0 J/mol · K. What is the enthalpy of vaporization of water at its normal boiling point?
(Multiple Choice)
4.7/5  (40)
(40)
Showing 61 - 80 of 157
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)