Exam 18: Thermodynamics: Spontaneous and Nonspontaneous Reactions and Processes
Exam 1: Matter and Energy: The Origin of the Universe99 Questions
Exam 2: Atoms, Ions, and Molecules: Matter Starts Here131 Questions
Exam 3: Stoichiometry: Mass, Formulas, and Reactions133 Questions
Exam 4: Solution Chemistry: The Hydrosphere126 Questions
Exam 5: Thermochemistry: Energy Changes in Reactions132 Questions
Exam 6: Properties of Gases: the Air We Breathe138 Questions
Exam 7: A Quantum Model of Atoms: Waves and Particles143 Questions
Exam 8: Chemical Bonds: What Makes a Gas a Greenhouse Gas139 Questions
Exam 9: Molecular Geometry: Shape Determines Function136 Questions
Exam 10: Intermolecular Forces: The Uniqueness of Water140 Questions
Exam 11: Solutions: Properties and Behavior130 Questions
Exam 12: Solids: Structures and Applications144 Questions
Exam 13: Organic Chemistry: Fuels, Pharmaceuticals, Materials, and Life129 Questions
Exam 14: Chemical Kinetics: Reactions in the Air We Breathe164 Questions
Exam 15: Chemical Equilibrium: How Much Product Does a Reaction Really Make91 Questions
Exam 16: Acid-Base and Solubility Equilibria: Reactions in Soil and Water179 Questions
Exam 17: Metal Ions: Colorful and Essential144 Questions
Exam 18: Thermodynamics: Spontaneous and Nonspontaneous Reactions and Processes157 Questions
Exam 19: Electrochemistry: the Quest for Clean Energy143 Questions
Exam 20: Biochemistry: the Compounds of Life108 Questions
Exam 21: Nuclear Chemistry: Applications to Energy and Medicine144 Questions
Exam 22: Life and the Periodic Table95 Questions
Select questions type
In an experiment, 1.00 mol of sodium metal is placed in a container and reacts with an excess of chlorine gas to form sodium chloride under standard state conditions. Determine Srxn given the following information: Substance (/ - K) (s) 51.45 (g) 222.96 (s) 72.33
(Multiple Choice)
4.8/5  (41)
(41)
Of the three modes of molecular motion-vibration, rotation, and translation-which requires the greatest amount of energy to cause an excitation from the ground state to the first excited state?
(Multiple Choice)
4.9/5  (36)
(36)
Only one substance has a standard entropy of 0 J/K, and that is H+(aq). Why is this so?
(Essay)
4.8/5  (35)
(35)
The standard entropy of N2(g) is 191.5 J/mol . K. Calculate the entropy per nitrogen molecule and the number of microstates for each molecule. Discuss the number of microstates in terms of the molecular motions accessible to each molecule.
(Essay)
4.8/5  (37)
(37)
A sketch of the free energy for a hypothetical chemical equilibrium is shown here. What part of the plot on the axis representing the relative quantities of reactants and products corresponds to a value of G that is greater than zero? 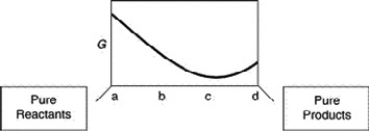
(Multiple Choice)
4.8/5  (44)
(44)
As the temperature of a reaction with S° > 0 is increased, the equilibrium constant ________
(Multiple Choice)
4.9/5  (36)
(36)
Which of the following will have the greatest standard molar entropy (S°)?
(Multiple Choice)
4.9/5  (32)
(32)
If 1 mol of ice melts at its melting point of 273 K, the entropy change for the ice is 22.0 J/K. If the ice melts in someone's hand at 34°C, what is the change in the entropy of the universe? Assume a final temperature for the water of 0°C. The enthalpy of fusion for ice is 6.01 kJ/mol.
(Multiple Choice)
4.9/5  (39)
(39)
As the temperature of an endothermic reaction is increased, the equilibrium constant ________
(Multiple Choice)
4.9/5  (36)
(36)
The reaction
Cr(NH3)63+(aq) + 3en(aq) Cr(en)33+(aq) + 6 NH3(aq)
Where en represents ethylenediamine, has a small value for the enthalpy change, Hrxn, yet the free-energy change is large because ________
(Multiple Choice)
4.9/5  (41)
(41)
Noxious NO gas can form from N2 and O2 gases in automobile engines at high temperatures. If the value of the equilibrium constant, Kc, for this reaction at 25°C is 1.95 *10-31, what is the value at 2000°C?
N2(g) + O2(g) 2NO(g)
Thermodynamic Properties of Nitrogen Monoxide \Delta \Delta 87.6/ 90.3/ 211/(\cdot)
(Multiple Choice)
4.9/5  (39)
(39)
Which of the following processes are reversible in the thermodynamic sense?
I. Iron in the open air rusts.
II. NaCl is dissolved in water and then recovered by the evaporation of the water.
III. The ice in a mixture of ice and water at 0°C and 1 atm melts.
(Multiple Choice)
4.7/5  (39)
(39)
A sealed tube containing an equilibrium mixture of NO2(g) (brown) and N2O4(g) (colorless) is subjected to a variety of temperatures. For the equilibrium reaction shown, the values of the H°rxn and S°rxn are also shown. What is observed in the tube with increasing temperature?
2NO2(g) N2O4(g) H = -58.02 kJ/mol, S=-176.5 J/mol . K
(Multiple Choice)
4.9/5  (38)
(38)
A reaction with a low enthalpy of reaction value is not spontaneous at low temperature but becomes spontaneous at high temperature. What are the signs for H° and S°, respectively?
(Multiple Choice)
4.9/5  (40)
(40)
Benzene is a liquid under standard conditions. The standard free-energy change for the evaporation of liquid benzene to form benzene vapor is +5.2 kJ/mol. If liquid benzene is placed in an open container under standard conditions, ________
(Multiple Choice)
4.8/5  (33)
(33)
Give an example of a spontaneous and a nonspontaneous chemical process and the sign of the entropy change for the universe for each.
(Essay)
4.8/5  (43)
(43)
Consider a reaction at a point in which the difference in free energy between the reactants and products is twice the standard free-energy change for the reaction. What expression would allow you to calculate the reaction quotient under these conditions, and what would be required for the reaction to be spontaneous?
(Short Answer)
4.9/5  (44)
(44)
The enthalpy and entropy of vaporization of ethanol are 38.6 kJ/mol and 109.8 J/mol · K, respectively. What is the boiling point of ethanol under equilibrium conditions, in °C?
(Multiple Choice)
4.9/5  (36)
(36)
Heat transfer from the system to the surroundings has a large effect on Ssurr ________
(Multiple Choice)
4.8/5  (43)
(43)
Showing 81 - 100 of 157
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)