Exam 18: Thermodynamics: Spontaneous and Nonspontaneous Reactions and Processes
Exam 1: Matter and Energy: The Origin of the Universe99 Questions
Exam 2: Atoms, Ions, and Molecules: Matter Starts Here131 Questions
Exam 3: Stoichiometry: Mass, Formulas, and Reactions133 Questions
Exam 4: Solution Chemistry: The Hydrosphere126 Questions
Exam 5: Thermochemistry: Energy Changes in Reactions132 Questions
Exam 6: Properties of Gases: the Air We Breathe138 Questions
Exam 7: A Quantum Model of Atoms: Waves and Particles143 Questions
Exam 8: Chemical Bonds: What Makes a Gas a Greenhouse Gas139 Questions
Exam 9: Molecular Geometry: Shape Determines Function136 Questions
Exam 10: Intermolecular Forces: The Uniqueness of Water140 Questions
Exam 11: Solutions: Properties and Behavior130 Questions
Exam 12: Solids: Structures and Applications144 Questions
Exam 13: Organic Chemistry: Fuels, Pharmaceuticals, Materials, and Life129 Questions
Exam 14: Chemical Kinetics: Reactions in the Air We Breathe164 Questions
Exam 15: Chemical Equilibrium: How Much Product Does a Reaction Really Make91 Questions
Exam 16: Acid-Base and Solubility Equilibria: Reactions in Soil and Water179 Questions
Exam 17: Metal Ions: Colorful and Essential144 Questions
Exam 18: Thermodynamics: Spontaneous and Nonspontaneous Reactions and Processes157 Questions
Exam 19: Electrochemistry: the Quest for Clean Energy143 Questions
Exam 20: Biochemistry: the Compounds of Life108 Questions
Exam 21: Nuclear Chemistry: Applications to Energy and Medicine144 Questions
Exam 22: Life and the Periodic Table95 Questions
Select questions type
Care must be taken when dissolving solid pellets of sodium hydroxide (NaOH) in water, because the temperature of the water can rise dramatically. Taking NaOH as the system, what can you deduce about the signs of the entropy change of the system ( Ssys) and surroundings ( Ssurr) from this?
(Multiple Choice)
4.7/5  (45)
(45)
In the equation relating equilibrium to thermodynamics, a = -RTb,
(Multiple Choice)
4.8/5  (41)
(41)
Which of the following must be true for a spontaneous exothermic process?
(Multiple Choice)
4.7/5  (35)
(35)
Which of the following is in the correct order of standard state entropy?
I.Diamond < graphite
II.Liquid water < solid water
III.NH3 < H2
(Multiple Choice)
4.8/5  (38)
(38)
Processes are always spontaneous when ________ (H and S refer to the system).
(Multiple Choice)
4.8/5  (36)
(36)
A sketch of the free energy for a hypothetical chemical equilibrium is shown here. What part of the plot on the axis representing the relative quantities of reactants and products corresponds to a value of Q that is less than K? 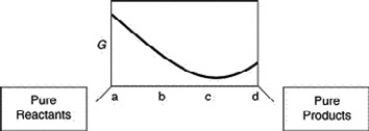
(Multiple Choice)
4.7/5  (34)
(34)
The following figures represent distributions of two types of gas molecules between two containers connected by an open tube. In which figure is the entropy of the system maximized?
(Multiple Choice)
4.9/5  (44)
(44)
Several groups of general chemistry lab students measured the equilibrium constant for the same chemical equilibrium. In comparing their results, they found that they had different values because the temperatures of the experiments were different. Everyone was disappointed by the inconsistency, except for Dexter when he realized the measurements were made at different measured temperatures: "My esteemed colleagues," he said, "together we have sufficient data to determine two additional thermodynamic parameters and show the lab instructor what we know!" What two parameters did Dexter have in mind?
(Multiple Choice)
4.8/5  (34)
(34)
Which of the following statements is/are correct? A large negative free-energy change for a reaction means that ________
I. the equilibrium constant for the reaction is large.
II. the equilibrium constant for the reaction is small.
III. the reaction greatly favors formation of the products.
IV. only a small amount of product is produced at equilibrium.
(Multiple Choice)
4.8/5  (47)
(47)
Suppose G° is 25.0 kJ/mol for a hypothetical reaction in which A converts into B. Which of the following statements describes an equilibrium mixture of A and B?
(Multiple Choice)
4.7/5  (31)
(31)
Hydrogen reacts with nitrogen to form ammonia (NH3) according to the reaction
3H2(g) + N2(g) 2NH3(g)
The value of H° is -92.38 kJ/mol, and that of S° is -198.2 J/(mol . K). Determine G° at 25°C.
(Multiple Choice)
4.9/5  (38)
(38)
What is the overall standard free-energy change for the following two reactions?
A + B 2C G
\circ rxn
= G1
C + D E G
\circ rxn
= G2
(Multiple Choice)
4.9/5  (37)
(37)
Calcium sulfate is a desiccant used for storage of samples and equipment in a dry atmosphere because it absorbs water from air. The relevant thermodynamic reaction equation is given below. At what temperature will this reaction reverse to release the water and regenerate the dry desiccant?
CaSO4(s) + 2H2O(g) CaSO4.H2O(s)
H° = -104.9 kJ/mol, S° = -291.2 J/(mol K)
(Multiple Choice)
4.8/5  (34)
(34)
Which of the following relationships are sufficient to ensure that reactants and products will be in their standard state at equilibrium?
I. G = 0
II. G° = 0
III. K = 1
(Multiple Choice)
4.9/5  (45)
(45)
Determine the entropy change for the reaction SO2(g) + O2(g) SO3(g) given the following information: Substance (/\cdot) (g) 248.2 (g) 205.0 (g) 256.8
(Multiple Choice)
4.8/5  (38)
(38)
Values for H° and S° for a reaction can be determined experimentally by fitting a linear equation to a plot of ________ on the x-axis and ________ on the y-axis. The slope of this equation is equal to ________ and the intercept allows us to calculate ________.
(Essay)
4.7/5  (32)
(32)
Because the triple point of water is 273 K, what must be true of the change in enthalpy and the change in entropy for ice converting to water at this temperature?
(Multiple Choice)
4.9/5  (36)
(36)
Hydrochloric acid (HCl) reacts with sodium hydroxide (NaOH) to form sodium chloride (NaCl)
And water. If H° = -56.13 kJ/mol and S° = 79.11 J/mol . K, what is G° for this reaction at 20°C?
(Multiple Choice)
4.8/5  (40)
(40)
Showing 121 - 140 of 157
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)