Exam 12: Solids: Structures and Applications
Exam 1: Matter and Energy: The Origin of the Universe99 Questions
Exam 2: Atoms, Ions, and Molecules: Matter Starts Here131 Questions
Exam 3: Stoichiometry: Mass, Formulas, and Reactions133 Questions
Exam 4: Solution Chemistry: The Hydrosphere126 Questions
Exam 5: Thermochemistry: Energy Changes in Reactions132 Questions
Exam 6: Properties of Gases: the Air We Breathe138 Questions
Exam 7: A Quantum Model of Atoms: Waves and Particles143 Questions
Exam 8: Chemical Bonds: What Makes a Gas a Greenhouse Gas139 Questions
Exam 9: Molecular Geometry: Shape Determines Function136 Questions
Exam 10: Intermolecular Forces: The Uniqueness of Water140 Questions
Exam 11: Solutions: Properties and Behavior130 Questions
Exam 12: Solids: Structures and Applications144 Questions
Exam 13: Organic Chemistry: Fuels, Pharmaceuticals, Materials, and Life129 Questions
Exam 14: Chemical Kinetics: Reactions in the Air We Breathe164 Questions
Exam 15: Chemical Equilibrium: How Much Product Does a Reaction Really Make91 Questions
Exam 16: Acid-Base and Solubility Equilibria: Reactions in Soil and Water179 Questions
Exam 17: Metal Ions: Colorful and Essential144 Questions
Exam 18: Thermodynamics: Spontaneous and Nonspontaneous Reactions and Processes157 Questions
Exam 19: Electrochemistry: the Quest for Clean Energy143 Questions
Exam 20: Biochemistry: the Compounds of Life108 Questions
Exam 21: Nuclear Chemistry: Applications to Energy and Medicine144 Questions
Exam 22: Life and the Periodic Table95 Questions
Select questions type
When Si is doped with P, it produces a(n) ________-type semiconductor.
(Short Answer)
4.7/5  (42)
(42)
X-ray diffraction (XRD) makes use of the Bragg equation in which is the ________
(Multiple Choice)
4.9/5  (29)
(29)
The alpha form of polonium (Po) crystallizes as a simple cubic unit cell with an edge length of
335 pm. What is the atomic radius of polonium?
(Multiple Choice)
4.8/5  (41)
(41)
In the cesium chloride unit cell shown below, the cesium ions sit on the corners of a cube. What is the name of this unit cell? 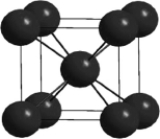
(Multiple Choice)
4.8/5  (37)
(37)
Iron (Fe) has a density of 7.874 g/cm3 and crystallizes in a body-centered cubic structure. What is the atomic radius of iron?
(Multiple Choice)
5.0/5  (40)
(40)
Which of the following can be varied to change the physical properties of an alloy?
I.The elements used
II.The proportions used
III.The type of hole each element occupies
(Multiple Choice)
4.8/5  (32)
(32)
Sphalerite (ZnS) is the chief ore of zinc. In the unit cell of sphalerite, ________
(Multiple Choice)
4.9/5  (37)
(37)
How many nearest neighbor atoms are there around each atom in a simple cubic unit cell?
(Multiple Choice)
4.9/5  (31)
(31)
The thermal and electrical insulating qualities of ceramics can be explained by ________
(Multiple Choice)
4.8/5  (37)
(37)
The alpha form of polonium (Po) crystallizes as a simple cubic unit cell with an edge length of 335 pm. What is the atomic radius of polonium in picometers?
(Short Answer)
4.7/5  (48)
(48)
In a simple cubic unit cell there is/are ________ atom(s), in a face-centered cubic cell there is/are ________ atom(s), and in a body-centered cubic cell there is/are ________ atom(s).
(Short Answer)
4.8/5  (37)
(37)
The face-centered cubic unit cell has ________ tetrahedral holes.
(Multiple Choice)
4.8/5  (38)
(38)
If a face-centered cubic unit cell has a volume of 1.447* 108 pm3 and the ions at the corners touch the ion on the face, what must be the ion's radius?
(Multiple Choice)
4.9/5  (48)
(48)
A ceramic is a chemically resistant and heat-resistant solid produced by heating ________
(Multiple Choice)
4.8/5  (37)
(37)
What is the likely unit cell for ionic compounds of 1:1 stoichiometry in which the difference in the radii is only about 25% or less?
(Multiple Choice)
4.7/5  (43)
(43)
The bonding in solid-state metals can be described as ________
(Multiple Choice)
4.8/5  (35)
(35)
Iron (Fe) crystallizes as a body-centered unit cell with an edge length of 287 pm. What is the atomic radius of iron in picometers?
(Short Answer)
4.8/5  (42)
(42)
The cubic closest-packed crystal structure has a ________ layering pattern that produces a ________ unit cell.
(Multiple Choice)
4.7/5  (38)
(38)
Showing 21 - 40 of 144
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)