Exam 12: Solids: Structures and Applications
Exam 1: Matter and Energy: The Origin of the Universe99 Questions
Exam 2: Atoms, Ions, and Molecules: Matter Starts Here131 Questions
Exam 3: Stoichiometry: Mass, Formulas, and Reactions133 Questions
Exam 4: Solution Chemistry: The Hydrosphere126 Questions
Exam 5: Thermochemistry: Energy Changes in Reactions132 Questions
Exam 6: Properties of Gases: the Air We Breathe138 Questions
Exam 7: A Quantum Model of Atoms: Waves and Particles143 Questions
Exam 8: Chemical Bonds: What Makes a Gas a Greenhouse Gas139 Questions
Exam 9: Molecular Geometry: Shape Determines Function136 Questions
Exam 10: Intermolecular Forces: The Uniqueness of Water140 Questions
Exam 11: Solutions: Properties and Behavior130 Questions
Exam 12: Solids: Structures and Applications144 Questions
Exam 13: Organic Chemistry: Fuels, Pharmaceuticals, Materials, and Life129 Questions
Exam 14: Chemical Kinetics: Reactions in the Air We Breathe164 Questions
Exam 15: Chemical Equilibrium: How Much Product Does a Reaction Really Make91 Questions
Exam 16: Acid-Base and Solubility Equilibria: Reactions in Soil and Water179 Questions
Exam 17: Metal Ions: Colorful and Essential144 Questions
Exam 18: Thermodynamics: Spontaneous and Nonspontaneous Reactions and Processes157 Questions
Exam 19: Electrochemistry: the Quest for Clean Energy143 Questions
Exam 20: Biochemistry: the Compounds of Life108 Questions
Exam 21: Nuclear Chemistry: Applications to Energy and Medicine144 Questions
Exam 22: Life and the Periodic Table95 Questions
Select questions type
Aluminum (Al) has a density of 2.70 g/cm3 and crystallizes in a face-centered cubic structure. What is the unit cell edge length?
(Multiple Choice)
4.8/5  (35)
(35)
A face-centered cubic unit cell has a(n) ________ in its center.
(Multiple Choice)
4.8/5  (33)
(33)
The face-centered cubic structure is also known as ________
(Multiple Choice)
4.8/5  (41)
(41)
When Ge is doped with Ga, it produces a(n) ________-type semiconductor.
(Multiple Choice)
4.9/5  (47)
(47)
If an X-ray with a wavelength of 154 pm is diffracted at an angle 2 = 14.6°, according to the Bragg equation (n = 2 d sin ), what is the distance between layers of the crystal that give rise to this diffraction? Assume n = 1 in this problem.
(Multiple Choice)
4.7/5  (35)
(35)
Bronze that is composed of 10% tin and 90% copper is ________
(Multiple Choice)
4.7/5  (38)
(38)
How many nearest neighbor atoms are there around each atom in a body-centered cubic unit cell?
(Multiple Choice)
4.7/5  (35)
(35)
At a historic Civil War battleground, a stack of cannonballs looked like the picture below on the far left. Removing the top cannonball resulted in the middle view, and removing the next layer resulted in the view on the right. What sort of packing was used in stacking the cannonballs? 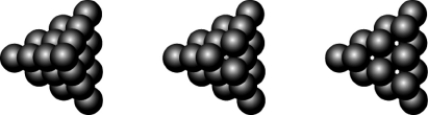
(Multiple Choice)
4.9/5  (39)
(39)
Metal solids are good conductors of electricity because ________
(Multiple Choice)
4.9/5  (37)
(37)
Which statement A-D does not describe a simple cubic unit cell?
(Multiple Choice)
4.8/5  (38)
(38)
Which statement regarding the formation of an alloy is correct?
(Multiple Choice)
4.9/5  (33)
(33)
Which element would be used to dope germanium to produce an n-type semiconductor?
(Multiple Choice)
4.9/5  (43)
(43)
The hexagonal closest-packed crystal structure has a ________ layering pattern that produces a ________ unit cell.
(Multiple Choice)
4.8/5  (40)
(40)
Ionic solids have ________ melting points, and are ________.
(Multiple Choice)
4.8/5  (39)
(39)
When Ge is doped with Ga, it produces a(n) ________-type semiconductor.
(Short Answer)
4.8/5  (44)
(44)
The property of superconducting ceramics that makes them a potential technology for levitating trains is ________
(Multiple Choice)
4.9/5  (29)
(29)
Showing 101 - 120 of 144
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)