Exam 12: Solids: Structures and Applications
Exam 1: Matter and Energy: The Origin of the Universe99 Questions
Exam 2: Atoms, Ions, and Molecules: Matter Starts Here131 Questions
Exam 3: Stoichiometry: Mass, Formulas, and Reactions133 Questions
Exam 4: Solution Chemistry: The Hydrosphere126 Questions
Exam 5: Thermochemistry: Energy Changes in Reactions132 Questions
Exam 6: Properties of Gases: the Air We Breathe138 Questions
Exam 7: A Quantum Model of Atoms: Waves and Particles143 Questions
Exam 8: Chemical Bonds: What Makes a Gas a Greenhouse Gas139 Questions
Exam 9: Molecular Geometry: Shape Determines Function136 Questions
Exam 10: Intermolecular Forces: The Uniqueness of Water140 Questions
Exam 11: Solutions: Properties and Behavior130 Questions
Exam 12: Solids: Structures and Applications144 Questions
Exam 13: Organic Chemistry: Fuels, Pharmaceuticals, Materials, and Life129 Questions
Exam 14: Chemical Kinetics: Reactions in the Air We Breathe164 Questions
Exam 15: Chemical Equilibrium: How Much Product Does a Reaction Really Make91 Questions
Exam 16: Acid-Base and Solubility Equilibria: Reactions in Soil and Water179 Questions
Exam 17: Metal Ions: Colorful and Essential144 Questions
Exam 18: Thermodynamics: Spontaneous and Nonspontaneous Reactions and Processes157 Questions
Exam 19: Electrochemistry: the Quest for Clean Energy143 Questions
Exam 20: Biochemistry: the Compounds of Life108 Questions
Exam 21: Nuclear Chemistry: Applications to Energy and Medicine144 Questions
Exam 22: Life and the Periodic Table95 Questions
Select questions type
According to band theory, when the lower energy ________ band is separated from the higher energy ________ band by a large band gap, the material will act as a ________ of electricity.
(Short Answer)
4.8/5  (40)
(40)
If a body-centered cubic unit cell has a volume of 1.447 *108 pm3, what must be the dimension of the cube's edge in picometers?
(Short Answer)
4.8/5  (41)
(41)
Gold has a face-centered cubic structure with a unit cell edge length of 407.8 pm. What is the contribution to the density from each individual gold atom in g/cm3?
(Short Answer)
4.8/5  (40)
(40)
Why are ceramic superconductors not currently practical for widespread transmission of electricity?
I.They are relatively expensive in comparison to metals.
II.They require extremely cold temperatures.
III.They are brittle and lack ductility.
(Multiple Choice)
4.8/5  (38)
(38)
Which of the following unit cells has the lowest packing efficiency?
(Multiple Choice)
4.8/5  (36)
(36)
Which of the following unit cells has the highest packing efficiency?
(Multiple Choice)
4.9/5  (37)
(37)
The XRD scan of the face-centered cubic (fcc) structure of sodium chloride showed that there was a distance of 562.8 pm between "layers of ions." Given that the fcc unit cell has a volume of 178.26 *10-24 cm3, to which distance does the 562.8 pm correspond?
(Multiple Choice)
4.9/5  (43)
(43)
Firing of a kaolinite clay object to make a ceramic results in ________
(Multiple Choice)
4.9/5  (35)
(35)
The alpha form of polonium (Po) has a density of 9.196 g/cm3 and crystallizes in a simple cubic structure. What is the atomic radius of polonium?
(Multiple Choice)
4.8/5  (32)
(32)
An approximately spherical allotrope of carbon containing 60 or 70 atoms is ________
(Multiple Choice)
4.9/5  (29)
(29)
In ionic solids, one ion often occupies a hole in the unit cell defined by the other ion. In a body-centered cubic cell, what are the types and numbers of each hole?
(Short Answer)
4.9/5  (41)
(41)
Iron (Fe) crystallizes as a body-centered unit cell with an edge length of 287 pm. What is the atomic radius of iron?
(Multiple Choice)
4.9/5  (38)
(38)
Consider the three unit cells below for ionic solids. In each unit cell, one of the ions is arranged in one of the standard unit cell structures while the other is found between these sites. What are the names of the minerals associated with each of these structures? I ________; II ________;
III ________. 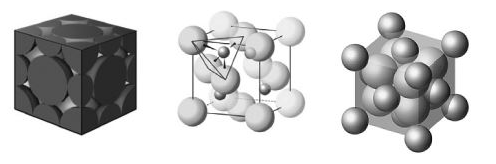 I II III In addition to the ions There are 14 large and There are 14 small and shown, there is another 4 small ions depicted. 8 large ions depicted. at the very center of the unit cell.
I II III In addition to the ions There are 14 large and There are 14 small and shown, there is another 4 small ions depicted. 8 large ions depicted. at the very center of the unit cell.
(Short Answer)
4.9/5  (32)
(32)
Which element would be used to dope silicon to produce a p-type semiconductor?
(Multiple Choice)
4.7/5  (37)
(37)
The closest packing of spheres (such as oranges, cannonballs, or atoms) has the spheres ________
(Multiple Choice)
4.9/5  (37)
(37)
In the solid-state structure of sodium chloride, the closest distance between the centers of ions is observed ________
(Multiple Choice)
4.9/5  (36)
(36)
A hexagonal closest-packed structure has hexagonally arranged layers of atoms stacking in the series ________
(Multiple Choice)
4.9/5  (33)
(33)
Iron crystallizes in a body-centered cubic pattern. How many iron atoms are in each unit cell?
(Multiple Choice)
4.8/5  (39)
(39)
Showing 121 - 140 of 144
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)