Exam 12: Solids: Structures and Applications
Exam 1: Matter and Energy: The Origin of the Universe99 Questions
Exam 2: Atoms, Ions, and Molecules: Matter Starts Here131 Questions
Exam 3: Stoichiometry: Mass, Formulas, and Reactions133 Questions
Exam 4: Solution Chemistry: The Hydrosphere126 Questions
Exam 5: Thermochemistry: Energy Changes in Reactions132 Questions
Exam 6: Properties of Gases: the Air We Breathe138 Questions
Exam 7: A Quantum Model of Atoms: Waves and Particles143 Questions
Exam 8: Chemical Bonds: What Makes a Gas a Greenhouse Gas139 Questions
Exam 9: Molecular Geometry: Shape Determines Function136 Questions
Exam 10: Intermolecular Forces: The Uniqueness of Water140 Questions
Exam 11: Solutions: Properties and Behavior130 Questions
Exam 12: Solids: Structures and Applications144 Questions
Exam 13: Organic Chemistry: Fuels, Pharmaceuticals, Materials, and Life129 Questions
Exam 14: Chemical Kinetics: Reactions in the Air We Breathe164 Questions
Exam 15: Chemical Equilibrium: How Much Product Does a Reaction Really Make91 Questions
Exam 16: Acid-Base and Solubility Equilibria: Reactions in Soil and Water179 Questions
Exam 17: Metal Ions: Colorful and Essential144 Questions
Exam 18: Thermodynamics: Spontaneous and Nonspontaneous Reactions and Processes157 Questions
Exam 19: Electrochemistry: the Quest for Clean Energy143 Questions
Exam 20: Biochemistry: the Compounds of Life108 Questions
Exam 21: Nuclear Chemistry: Applications to Energy and Medicine144 Questions
Exam 22: Life and the Periodic Table95 Questions
Select questions type
Researchers at the University of Texas at Austin prepared a linear allotrope of carbon in 1998. What is the hybridization of its atomic orbitals?
(Multiple Choice)
4.8/5  (38)
(38)
Which two of the following elements are found as a covalent network solid?
I.beryllium
II.carbon
III.phosphorus
IV.sulfur
(Multiple Choice)
5.0/5  (31)
(31)
In addition to carbon and iron, stainless steel contains ________
(Multiple Choice)
4.8/5  (35)
(35)
In the Bragg equation, refers to the angle of ________ or ________and 2 refers to the angle of ________.
(Short Answer)
4.9/5  (35)
(35)
Polonium crystallizes in a simple cubic pattern. How many polonium atoms are in each unit cell?
(Multiple Choice)
4.9/5  (31)
(31)
Graphite and diamond are examples of ________ solids; sulfur and white phosphorus are examples of ________ solids; and sodium chloride and zinc sulfide are examples of ________ solids.
(Short Answer)
4.8/5  (38)
(38)
In the sodium chloride unit cell, the chloride ions form a cube in which each side is arranged like the following figure. The circles represent the positions of the chloride ions on one square face of the cube. All the other faces are the same. What is the name of this unit cell? 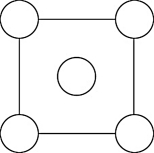
(Multiple Choice)
4.9/5  (42)
(42)
How many tennis balls will fit within the interstitial holes between a truckload of basketballs perfectly placed in a closest-packed arrangement? Assume that the tennis balls have a radius that is 20% that of a basketball.
(Multiple Choice)
4.8/5  (33)
(33)
The molecular orbital description for metal bonding is different from that for diatomic molecules in that ________
(Multiple Choice)
4.9/5  (41)
(41)
Which type of solid is held together by relatively weak dispersion forces?
(Multiple Choice)
4.8/5  (40)
(40)
Which two of the following elements are not found as a covalent network solid?
I.beryllium
II.carbon
III.phosphorus
IV.sulfur
(Multiple Choice)
4.9/5  (38)
(38)
Glass is a term used to describe any solid that is ________
(Multiple Choice)
4.7/5  (42)
(42)
In a two-component alloy the more abundant metal can be thought of as the solvent while the less abundant metal can be thought of as the solute. Which of the following would not change the position of atoms in the solvent's unit cell?
I.a solute with the same atomic radius as the solvent
II.a solute that was sufficiently small to fit into holes in the solvent's unit cell
III.a solvent that was sufficiently small to fit into holes in the solute's unit cell
(Multiple Choice)
4.9/5  (38)
(38)
Aluminum (Al) has a density of 2.70 g/cm3 and crystallizes in a face-centered cubic structure. What is the unit cell edge length in picometers?
(Short Answer)
4.8/5  (33)
(33)
Which of the following is true regarding the attractive force that holds sodium chloride in the solid state?
I.It is electrostatic.
II.It is termed ionic bonding.
III.It depends on the distance between the sodium and chloride.
IV.It only operates between adjacent sodium and chloride.
(Multiple Choice)
4.8/5  (44)
(44)
Showing 81 - 100 of 144
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)