Exam 10: Intermolecular Forces: The Uniqueness of Water
Exam 1: Matter and Energy: The Origin of the Universe99 Questions
Exam 2: Atoms, Ions, and Molecules: Matter Starts Here131 Questions
Exam 3: Stoichiometry: Mass, Formulas, and Reactions133 Questions
Exam 4: Solution Chemistry: The Hydrosphere126 Questions
Exam 5: Thermochemistry: Energy Changes in Reactions132 Questions
Exam 6: Properties of Gases: the Air We Breathe138 Questions
Exam 7: A Quantum Model of Atoms: Waves and Particles143 Questions
Exam 8: Chemical Bonds: What Makes a Gas a Greenhouse Gas139 Questions
Exam 9: Molecular Geometry: Shape Determines Function136 Questions
Exam 10: Intermolecular Forces: The Uniqueness of Water140 Questions
Exam 11: Solutions: Properties and Behavior130 Questions
Exam 12: Solids: Structures and Applications144 Questions
Exam 13: Organic Chemistry: Fuels, Pharmaceuticals, Materials, and Life129 Questions
Exam 14: Chemical Kinetics: Reactions in the Air We Breathe164 Questions
Exam 15: Chemical Equilibrium: How Much Product Does a Reaction Really Make91 Questions
Exam 16: Acid-Base and Solubility Equilibria: Reactions in Soil and Water179 Questions
Exam 17: Metal Ions: Colorful and Essential144 Questions
Exam 18: Thermodynamics: Spontaneous and Nonspontaneous Reactions and Processes157 Questions
Exam 19: Electrochemistry: the Quest for Clean Energy143 Questions
Exam 20: Biochemistry: the Compounds of Life108 Questions
Exam 21: Nuclear Chemistry: Applications to Energy and Medicine144 Questions
Exam 22: Life and the Periodic Table95 Questions
Select questions type
Which of the following compounds would be most soluble in carbon tetrachloride, CCl4?
(Multiple Choice)
4.9/5  (39)
(39)
Arrange the following compounds in order of increasing dispersion interactions: CCl4, CH4, C3H8.
(Multiple Choice)
4.8/5  (40)
(40)
The relative energies (strengths) of the intermolecular forces present in each of four different pure substances are shown in the figure below. Which substance has the highest melting point? 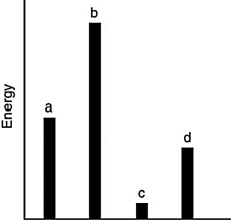
(Multiple Choice)
4.8/5  (38)
(38)
Gasoline is primarily a mixture of hydrocarbons and is sold with an octane rating that is based on a comparison with the combustion properties of isooctane. Gasoline usually contains an isomer of isooctane called tetramethylbutane (C8H18), which has an enthalpy of vaporization of 43.3 kJ/mol and a boiling point of 106.5°C. Determine the vapor pressure of tetramethylbutane on a very hot summer day when the temperature is 38°C.
(Multiple Choice)
4.8/5  (32)
(32)
Coulomb's law states that the energy of attraction between ions depends ________
(Multiple Choice)
4.7/5  (41)
(41)
A hydration sphere forms around an ion in aqueous solution due to ________
(Multiple Choice)
5.0/5  (37)
(37)
Consider the phase diagram for a substance shown here. The line between the solid and liquid phases has a positive slope because the solid phase is ________ than the liquid phase. 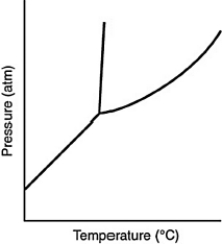
(Multiple Choice)
4.8/5  (35)
(35)
Which of the following substances is a solid at 25°C and 1 atm?
(Multiple Choice)
4.8/5  (46)
(46)
The relative energies (strengths) of the intermolecular forces present in each of four different pure gases are shown in the figure below. Which gas will show the smallest deviation from ideal gas behavior? 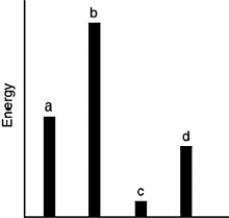
(Multiple Choice)
4.8/5  (36)
(36)
The density of water decreases as it is cooled from 4.0°C to freezing because ________
(Multiple Choice)
4.8/5  (44)
(44)
A phase diagram shows the states of a substance as a function of ________ and ________.
(Multiple Choice)
4.8/5  (32)
(32)
Which statement about the phase diagram below is not correct? 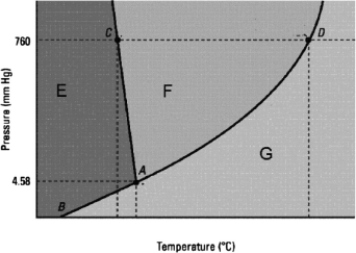
(Multiple Choice)
5.0/5  (28)
(28)
Gasoline is primarily a mixture of hydrocarbons and is sold with an octane rating that is based on a comparison with the properties of isooctane (C8H18), which has an enthalpy of vaporization of 35.8 kJ/mol and a boiling point of 98.2°C. Determine the vapor pressure of isooctane on a very hot summer day when the temperature is 38°C.
(Multiple Choice)
4.9/5  (31)
(31)
The relative energies (strengths) of the intermolecular forces between four different substances are shown in the figure below. Which substance has the lowest boiling point? 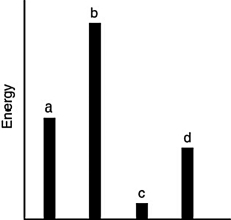
(Multiple Choice)
4.8/5  (35)
(35)
Why do the strengths of dispersion interactions generally increase with the molar mass of the compound?
(Essay)
4.8/5  (36)
(36)
Showing 21 - 40 of 140
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)