Exam 10: Intermolecular Forces: The Uniqueness of Water
Exam 1: Matter and Energy: The Origin of the Universe99 Questions
Exam 2: Atoms, Ions, and Molecules: Matter Starts Here131 Questions
Exam 3: Stoichiometry: Mass, Formulas, and Reactions133 Questions
Exam 4: Solution Chemistry: The Hydrosphere126 Questions
Exam 5: Thermochemistry: Energy Changes in Reactions132 Questions
Exam 6: Properties of Gases: the Air We Breathe138 Questions
Exam 7: A Quantum Model of Atoms: Waves and Particles143 Questions
Exam 8: Chemical Bonds: What Makes a Gas a Greenhouse Gas139 Questions
Exam 9: Molecular Geometry: Shape Determines Function136 Questions
Exam 10: Intermolecular Forces: The Uniqueness of Water140 Questions
Exam 11: Solutions: Properties and Behavior130 Questions
Exam 12: Solids: Structures and Applications144 Questions
Exam 13: Organic Chemistry: Fuels, Pharmaceuticals, Materials, and Life129 Questions
Exam 14: Chemical Kinetics: Reactions in the Air We Breathe164 Questions
Exam 15: Chemical Equilibrium: How Much Product Does a Reaction Really Make91 Questions
Exam 16: Acid-Base and Solubility Equilibria: Reactions in Soil and Water179 Questions
Exam 17: Metal Ions: Colorful and Essential144 Questions
Exam 18: Thermodynamics: Spontaneous and Nonspontaneous Reactions and Processes157 Questions
Exam 19: Electrochemistry: the Quest for Clean Energy143 Questions
Exam 20: Biochemistry: the Compounds of Life108 Questions
Exam 21: Nuclear Chemistry: Applications to Energy and Medicine144 Questions
Exam 22: Life and the Periodic Table95 Questions
Select questions type
For each of the following pairs of compounds, identify the one that is more likely to be soluble in water. Explain the rationale for your choice.
(A) Br2 or NaBr
(B) CH3CH2OH or CH3OCH3
(C) CO2 or KOH
(Essay)
4.8/5  (33)
(33)
Which one of the following substances would you predict to have the highest vapor pressure at a given temperature? In these line drawings, a carbon is implicit at the end of a line (bond) or where two or more lines come together. Carbon requires four bonds, so any missing bonds are implicitly bonds to hydrogen atoms.
(Multiple Choice)
4.8/5  (36)
(36)
The smell of fresh-cut pine is due in part to a cyclic alkene called pinene. A graph of the natural logarithm of the vapor pressure of pinene vs. 1/temperature produces a straight line with a slope of -4936.37 K. What is the enthalpy of vaporization of pinene?
(Multiple Choice)
4.8/5  (34)
(34)
The temperature at point a in the phase diagram below is the ________ 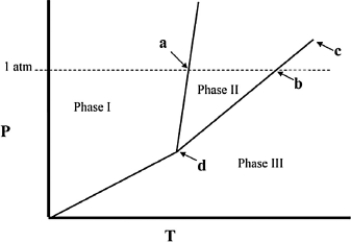
(Multiple Choice)
4.9/5  (35)
(35)
Which one of the following substances would you predict to have the highest boiling point? In these line drawings, a carbon is implicit at the end of a line (bond) or where two or more lines come together. Carbon requires four bonds, so any missing bonds are implicitly bonds to hydrogen atoms.
(Multiple Choice)
4.7/5  (31)
(31)
On the phase diagram below, identify the normal boiling point. 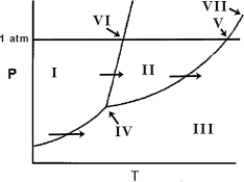
(Multiple Choice)
4.8/5  (39)
(39)
The phase diagram for carbon dioxide is shown below. What is the phase that exists at room temperature and pressure (22oC, 1 atm)? 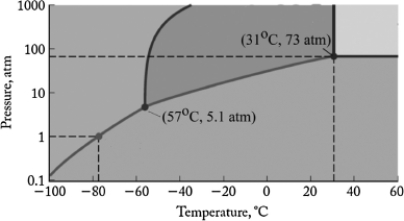
(Multiple Choice)
5.0/5  (36)
(36)
Why does HI boil at a higher temperature than HBr, yet the dipole moment of HBr (0.82 D) is larger than that of HI (0.38 D)?
(Essay)
4.9/5  (38)
(38)
Indicate which of the following molecules exhibits the greatest dispersion forces.
(Multiple Choice)
4.9/5  (35)
(35)
Which of the following pairs of compounds is most likely to be immiscible?
(Multiple Choice)
4.8/5  (35)
(35)
Under similar conditions, which of the following gases will behave less like an ideal gas than the others?
(Multiple Choice)
4.7/5  (42)
(42)
Which of the following substances has a solid whose freezing point will decrease with increasing pressure?
(Multiple Choice)
4.8/5  (39)
(39)
Which of the following will have the largest cation-anion attraction?
(Multiple Choice)
4.8/5  (36)
(36)
Indicate which of the following nonpolar compounds will have the lowest boiling point.
(Multiple Choice)
5.0/5  (31)
(31)
Carbon dioxide is being used as an environmentally safe liquid solvent for reactions. If the reaction is run at a temperature of 15oC, what must the minimum pressure of the reaction vessel be? The phase diagram for carbon dioxide is shown below. 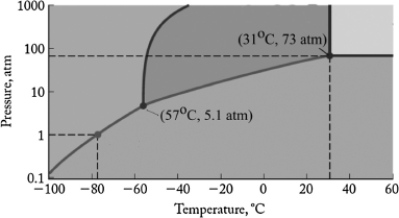
(Multiple Choice)
4.9/5  (39)
(39)
Which of the following polar compounds is likely to have the highest boiling point?
(Multiple Choice)
4.9/5  (34)
(34)
Showing 121 - 140 of 140
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)