Exam 10: Intermolecular Forces: The Uniqueness of Water
Exam 1: Matter and Energy: The Origin of the Universe99 Questions
Exam 2: Atoms, Ions, and Molecules: Matter Starts Here131 Questions
Exam 3: Stoichiometry: Mass, Formulas, and Reactions133 Questions
Exam 4: Solution Chemistry: The Hydrosphere126 Questions
Exam 5: Thermochemistry: Energy Changes in Reactions132 Questions
Exam 6: Properties of Gases: the Air We Breathe138 Questions
Exam 7: A Quantum Model of Atoms: Waves and Particles143 Questions
Exam 8: Chemical Bonds: What Makes a Gas a Greenhouse Gas139 Questions
Exam 9: Molecular Geometry: Shape Determines Function136 Questions
Exam 10: Intermolecular Forces: The Uniqueness of Water140 Questions
Exam 11: Solutions: Properties and Behavior130 Questions
Exam 12: Solids: Structures and Applications144 Questions
Exam 13: Organic Chemistry: Fuels, Pharmaceuticals, Materials, and Life129 Questions
Exam 14: Chemical Kinetics: Reactions in the Air We Breathe164 Questions
Exam 15: Chemical Equilibrium: How Much Product Does a Reaction Really Make91 Questions
Exam 16: Acid-Base and Solubility Equilibria: Reactions in Soil and Water179 Questions
Exam 17: Metal Ions: Colorful and Essential144 Questions
Exam 18: Thermodynamics: Spontaneous and Nonspontaneous Reactions and Processes157 Questions
Exam 19: Electrochemistry: the Quest for Clean Energy143 Questions
Exam 20: Biochemistry: the Compounds of Life108 Questions
Exam 21: Nuclear Chemistry: Applications to Energy and Medicine144 Questions
Exam 22: Life and the Periodic Table95 Questions
Select questions type
The boiling point of HBr is higher than that of HCl because HBr has ________ (The dipole moments are 1.08 D for HCl and 0.82 D for HBr.)
(Multiple Choice)
4.9/5  (36)
(36)
Which hydride do you predict has the highest boiling point?
(Multiple Choice)
4.9/5  (45)
(45)
CH2F2 has a dipole moment of 1.93 D and a boiling point of -52°C. CH2Cl2 has a dipole moment of 1.60 D and a boiling point of 40°C. Why is the boiling point of dichloromethane so much higher than that of difluoromethane?
(Essay)
4.9/5  (27)
(27)
Which of the following substances has a solid that is less dense than the liquid?
(Multiple Choice)
4.9/5  (45)
(45)
Which is the dominant interaction between acetone molecules, (CH3)2CO?
(Multiple Choice)
4.9/5  (40)
(40)
On the phase diagram below, identify the normal melting point. 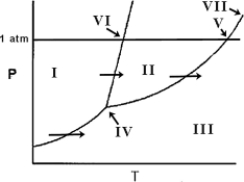
(Multiple Choice)
4.8/5  (33)
(33)
Which of the following diagrams best shows a set of polar molecules interacting through dipole-dipole interactions?
(Multiple Choice)
4.7/5  (41)
(41)
Arrange the three ionic compounds sodium chloride, magnesium chloride, and aluminum chloride in order of increasing melting point.
(Multiple Choice)
4.8/5  (36)
(36)
The relative energies (strengths) of the intermolecular forces present in each of four different pure substances are shown in the figure below. Which substance is most likely to be a gas at room temperature? 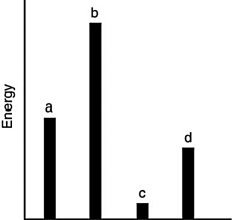
(Multiple Choice)
4.9/5  (36)
(36)
Which is the dominant interaction that leads to the formation of a hydration sphere in a salt solution?
(Multiple Choice)
4.9/5  (31)
(31)
Would water rise to the same height in a capillary tube made of polyethylene plastic as it does in a glass capillary tube of the same diameter? Polyethylene is a hydrocarbon, and glass is mostly silicon dioxide. Explain.
(Essay)
4.9/5  (41)
(41)
Indicate which of the following pairs of compounds is most likely to be miscible.
(Multiple Choice)
4.7/5  (33)
(33)
The phase diagram for carbon dioxide is shown below. What are the phase changes in order as carbon dioxide is heated from -90oC to 50oC, at 1 atm pressure? 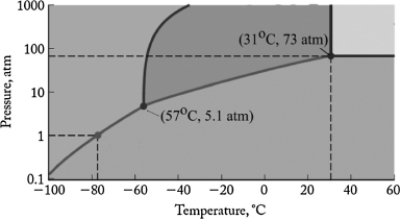
(Multiple Choice)
4.8/5  (33)
(33)
Ion interaction energies are determined by Coulomb's law: Determine the energy of potassium chloride if the radius of the potassium ion is 133 pm and that of the chloride ion is 181 pm.
(Multiple Choice)
4.9/5  (47)
(47)
What structural characteristics must a molecule have in order to hydrogen-bond?
(Essay)
4.9/5  (40)
(40)
Of all the noble gases, ________ has the weakest intermolecular force and hence the lowest boiling point.
(Multiple Choice)
4.7/5  (36)
(36)
For molecules or atoms with the same mass, which of the following typically is the weakest intermolecular interaction?
(Multiple Choice)
4.8/5  (41)
(41)
The aroma from almonds and cherries is due in part to a compound called benzaldehyde. A graph of the natural logarithm of the vapor pressure of benzaldehyde vs. 1/temperature produces a straight line with a slope of -5870.99 K. What is the enthalpy of vaporization of benzaldehyde?
(Multiple Choice)
4.7/5  (36)
(36)
Which of the following substances would you expect to have the largest van der Waals a constant value?
(Multiple Choice)
4.9/5  (36)
(36)
Showing 81 - 100 of 140
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)