Exam 7: A Quantum Model of Atoms: Waves and Particles
Exam 1: Matter and Energy: The Origin of the Universe99 Questions
Exam 2: Atoms, Ions, and Molecules: Matter Starts Here131 Questions
Exam 3: Stoichiometry: Mass, Formulas, and Reactions133 Questions
Exam 4: Solution Chemistry: The Hydrosphere126 Questions
Exam 5: Thermochemistry: Energy Changes in Reactions132 Questions
Exam 6: Properties of Gases: the Air We Breathe138 Questions
Exam 7: A Quantum Model of Atoms: Waves and Particles143 Questions
Exam 8: Chemical Bonds: What Makes a Gas a Greenhouse Gas139 Questions
Exam 9: Molecular Geometry: Shape Determines Function136 Questions
Exam 10: Intermolecular Forces: The Uniqueness of Water140 Questions
Exam 11: Solutions: Properties and Behavior130 Questions
Exam 12: Solids: Structures and Applications144 Questions
Exam 13: Organic Chemistry: Fuels, Pharmaceuticals, Materials, and Life129 Questions
Exam 14: Chemical Kinetics: Reactions in the Air We Breathe164 Questions
Exam 15: Chemical Equilibrium: How Much Product Does a Reaction Really Make91 Questions
Exam 16: Acid-Base and Solubility Equilibria: Reactions in Soil and Water179 Questions
Exam 17: Metal Ions: Colorful and Essential144 Questions
Exam 18: Thermodynamics: Spontaneous and Nonspontaneous Reactions and Processes157 Questions
Exam 19: Electrochemistry: the Quest for Clean Energy143 Questions
Exam 20: Biochemistry: the Compounds of Life108 Questions
Exam 21: Nuclear Chemistry: Applications to Energy and Medicine144 Questions
Exam 22: Life and the Periodic Table95 Questions
Select questions type
Which prediction regarding the formation of a monatomic ion most likely is not correct?
(Multiple Choice)
5.0/5  (33)
(33)
Which transition in a hydrogen atom will cause emission of the shortest wavelength photon?
(Multiple Choice)
4.9/5  (29)
(29)
A proton in a cyclotron has a velocity of 2 * 108 m/s, which is nearly the speed of light. The uncertainty in the velocity is 1%. What is the minimum uncertainty in the position of the proton in meters? The proton mass is 2 *10-27 kg.
(Multiple Choice)
4.7/5  (29)
(29)
The studies of light emitted by hot objects (Max Planck) and the photoelectric effect (Albert Einstein) revolutionized physics because they revealed ________
(Multiple Choice)
4.8/5  (42)
(42)
Identify which element, potassium or bromine, has the larger electron affinity and explain why.
(Essay)
4.8/5  (37)
(37)
Down a column in the periodic table, the ionization energy ________
(Multiple Choice)
4.7/5  (35)
(35)
Which statement about the quantum numbers that identify an atomic orbital is not correct?
(Multiple Choice)
4.8/5  (39)
(39)
The work function of sodium is 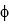 = 2.9 * 10-19 J. What is the maximum wavelength that can cause ejection of photoelectrons from a sodium surface?
= 2.9 * 10-19 J. What is the maximum wavelength that can cause ejection of photoelectrons from a sodium surface?
(Multiple Choice)
4.9/5  (37)
(37)
Indicate which of the following photons can cause emission of photoelectrons from a surface of gallium (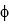 = 6.7 *10-19 J).
= 6.7 *10-19 J).
(Multiple Choice)
5.0/5  (43)
(43)
Early photoelectric light detectors were based on cesium. Cesium metal has a work function of 3.43 *10-19 J. What is the kinetic energy of the photoelectrons ejected from a cesium surface when exposed to blue light at 400 nm?
(Multiple Choice)
4.7/5  (35)
(35)
De Broglie proposed that = h/mv. Define the symbols in this equation and describe the significance of de Broglie's proposal.
(Essay)
4.9/5  (30)
(30)
According to de Broglie, if the circumference of the electron's orbit in the hydrogen atom is twice the electron's wavelength, the orbit will ________
(Multiple Choice)
4.9/5  (36)
(36)
Early photoelectric light detectors were based on cesium. Cesium metal has a work function of 3.43 *10-19 J. What is the velocity of the photoelectrons ejected from a cesium surface when exposed to blue light at 400 nm?
(Multiple Choice)
4.9/5  (43)
(43)
Which statement about refraction or diffraction is not correct?
(Multiple Choice)
4.8/5  (32)
(32)
Which of the following elements has the ground-state electron configuration 1s22s22p63s23p4?
(Multiple Choice)
4.8/5  (35)
(35)
What is the ground-state electron configuration of a Cl- ion?
(Multiple Choice)
4.8/5  (38)
(38)
The change in energy of a one-electron atom or ion for an electronic transition from the initial energy level ni to the final energy level nf is given by
E = -(2.18*10-18 J)
Z2
Which of the following species will have the longest wavelength emission line for the transition between the ni = 2 and nf = 1 levels?
(Multiple Choice)
4.7/5  (35)
(35)
Early photoelectric light detectors were based on cesium. Cesium metal has a work function of 3.43 *10-19 J. What is the minimum frequency of electromagnetic radiation required to eject photoelectrons from a cesium surface?
(Multiple Choice)
4.9/5  (35)
(35)
Which arrangement is in the correct order of increasing radii?
(Multiple Choice)
4.7/5  (47)
(47)
Showing 21 - 40 of 143
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)