Exam 7: A Quantum Model of Atoms: Waves and Particles
Exam 1: Matter and Energy: The Origin of the Universe99 Questions
Exam 2: Atoms, Ions, and Molecules: Matter Starts Here131 Questions
Exam 3: Stoichiometry: Mass, Formulas, and Reactions133 Questions
Exam 4: Solution Chemistry: The Hydrosphere126 Questions
Exam 5: Thermochemistry: Energy Changes in Reactions132 Questions
Exam 6: Properties of Gases: the Air We Breathe138 Questions
Exam 7: A Quantum Model of Atoms: Waves and Particles143 Questions
Exam 8: Chemical Bonds: What Makes a Gas a Greenhouse Gas139 Questions
Exam 9: Molecular Geometry: Shape Determines Function136 Questions
Exam 10: Intermolecular Forces: The Uniqueness of Water140 Questions
Exam 11: Solutions: Properties and Behavior130 Questions
Exam 12: Solids: Structures and Applications144 Questions
Exam 13: Organic Chemistry: Fuels, Pharmaceuticals, Materials, and Life129 Questions
Exam 14: Chemical Kinetics: Reactions in the Air We Breathe164 Questions
Exam 15: Chemical Equilibrium: How Much Product Does a Reaction Really Make91 Questions
Exam 16: Acid-Base and Solubility Equilibria: Reactions in Soil and Water179 Questions
Exam 17: Metal Ions: Colorful and Essential144 Questions
Exam 18: Thermodynamics: Spontaneous and Nonspontaneous Reactions and Processes157 Questions
Exam 19: Electrochemistry: the Quest for Clean Energy143 Questions
Exam 20: Biochemistry: the Compounds of Life108 Questions
Exam 21: Nuclear Chemistry: Applications to Energy and Medicine144 Questions
Exam 22: Life and the Periodic Table95 Questions
Select questions type
The emission spectra of Na and Na+ are different because ________
(Multiple Choice)
4.9/5  (35)
(35)
How many orbitals that have the principal quantum number equal to 3 are there in an atom?
(Multiple Choice)
4.9/5  (36)
(36)
Which of the following electron configurations represents an excited state?
(Multiple Choice)
4.7/5  (31)
(31)
What color will a yellow object appear when it is seen through a filter with the absorption spectrum shown below? 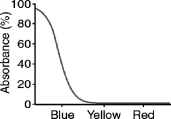
(Multiple Choice)
5.0/5  (42)
(42)
What is the orbital designation for an electron with the quantum numbers n = 3, = 2?
(Multiple Choice)
4.7/5  (37)
(37)
Astronomers have detected hydrogen atoms in interstellar space in the n = 732 energy level. Suppose an atom in this excited state emits a photon and undergoes a transition from n = 732 to n = 632. How much energy does the atom lose as a result of this transition? What is the frequency of this radiation? In which spectral region does this radiation lie?
(Essay)
4.8/5  (34)
(34)
Which of the following is the ground-state electron configuration of the Mg2+ ion?
(Multiple Choice)
4.8/5  (41)
(41)
Which of the following occurs only in discrete (quantized) increments?
(Multiple Choice)
4.9/5  (39)
(39)
Arrange the following elements in order of increasing first ionization energy:
3Li, 9F, 12Mg, 15P, 17Cl.
(Short Answer)
4.7/5  (37)
(37)
The size of a typical atomic orbital is on the order of ________
(Multiple Choice)
4.9/5  (47)
(47)
The atomic radius of germanium (Z = 32) is smaller than the atomic radius of potassium (Z = 19) because of ________
(Multiple Choice)
4.7/5  (34)
(34)
Across a row in the periodic table, the ionization energy ________
(Multiple Choice)
4.9/5  (31)
(31)
Which statement A-D about the wave function for a single electron is not correct?
(Multiple Choice)
4.8/5  (38)
(38)
If the principal quantum number is seven (n = 7) and the angular momentum quantum number is three ( = 3), which of the following values is not an allowed value of the magnetic quantum number (ml)?
(Multiple Choice)
4.8/5  (38)
(38)
An atom in its ground state absorbs a single photon of light and then relaxes back to the ground state by emitting an infrared photon (1,200 nm) followed by an orange photon (600 nm). What is the wavelength of the photon that was absorbed initially? 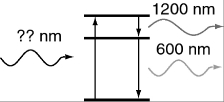
(Multiple Choice)
4.7/5  (41)
(41)
How many orbitals exist for the quantum numbers n = 4, = 1?
(Multiple Choice)
4.8/5  (32)
(32)
Which listing has the orbitals in order of increasing energy in a multielectron atom?
(Multiple Choice)
4.7/5  (30)
(30)
Showing 41 - 60 of 143
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)