Exam 7: A Quantum Model of Atoms: Waves and Particles
Exam 1: Matter and Energy: The Origin of the Universe99 Questions
Exam 2: Atoms, Ions, and Molecules: Matter Starts Here131 Questions
Exam 3: Stoichiometry: Mass, Formulas, and Reactions133 Questions
Exam 4: Solution Chemistry: The Hydrosphere126 Questions
Exam 5: Thermochemistry: Energy Changes in Reactions132 Questions
Exam 6: Properties of Gases: the Air We Breathe138 Questions
Exam 7: A Quantum Model of Atoms: Waves and Particles143 Questions
Exam 8: Chemical Bonds: What Makes a Gas a Greenhouse Gas139 Questions
Exam 9: Molecular Geometry: Shape Determines Function136 Questions
Exam 10: Intermolecular Forces: The Uniqueness of Water140 Questions
Exam 11: Solutions: Properties and Behavior130 Questions
Exam 12: Solids: Structures and Applications144 Questions
Exam 13: Organic Chemistry: Fuels, Pharmaceuticals, Materials, and Life129 Questions
Exam 14: Chemical Kinetics: Reactions in the Air We Breathe164 Questions
Exam 15: Chemical Equilibrium: How Much Product Does a Reaction Really Make91 Questions
Exam 16: Acid-Base and Solubility Equilibria: Reactions in Soil and Water179 Questions
Exam 17: Metal Ions: Colorful and Essential144 Questions
Exam 18: Thermodynamics: Spontaneous and Nonspontaneous Reactions and Processes157 Questions
Exam 19: Electrochemistry: the Quest for Clean Energy143 Questions
Exam 20: Biochemistry: the Compounds of Life108 Questions
Exam 21: Nuclear Chemistry: Applications to Energy and Medicine144 Questions
Exam 22: Life and the Periodic Table95 Questions
Select questions type
Which of the following elements would you expect to have the greatest first ionization energy?
(Multiple Choice)
4.8/5  (37)
(37)
Which of the following will lead to an increase in the kinetic energies of photoelectrons emitted when light is incident on a metal surface?
(Multiple Choice)
4.8/5  (37)
(37)
What is the speed of an argon atom that has a de Broglie wavelength of 5.2 pm?
(Multiple Choice)
4.9/5  (31)
(31)
Which of the transitions in the following hydrogen atom energy-level diagram involves the shortest wavelength photon? 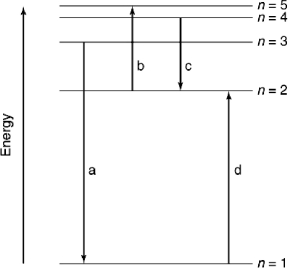
(Multiple Choice)
4.7/5  (35)
(35)
Give the names of the three quantum numbers (n,
, and m
) that identify the mathematical functions (orbitals) that are solutions to Schrödinger's wave equation, and describe how the energy, size, shape, and orientation of the orbital varies with the relative values of these quantum numbers.
(Essay)
4.9/5  (34)
(34)
Which atom or ion has the largest number of unpaired electrons?
(Multiple Choice)
4.7/5  (45)
(45)
What is the minimum energy that a photon must have to ionize a hydrogen atom from the n = 3 energy level?
(Short Answer)
4.8/5  (44)
(44)
De Broglie reasoned that for the electron in the hydrogen atom to behave as a stable circular wave, the circumference of the electron's orbit must be ________
(Multiple Choice)
4.9/5  (49)
(49)
What is the minimum frequency of a photon that can eject a photoelectron from Ba metal? (The work function of barium is 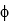 = 4.3 *10-19 J.)
= 4.3 *10-19 J.)
(Multiple Choice)
4.8/5  (41)
(41)
In refraction, polychromatic light is dispersed into its component wavelengths by ________
(Multiple Choice)
4.7/5  (42)
(42)
What is the frequency (v, in Hz) of the photons emitted by a He-Ne laser with a wavelength ( ) of 632.8 nm?
(Multiple Choice)
4.9/5  (43)
(43)
Which of the following metals would be an appropriate choice for the construction of a photon detector for 550 nm light?
(Multiple Choice)
4.8/5  (33)
(33)
How many of these atoms or ions have no unpaired electrons?
N, O, Na+, N3-, Sc
(Multiple Choice)
4.8/5  (41)
(41)
Copper forms a cation with a charge of 1+, but the element to the right of it in the periodic table (Zn) forms a cation with a 2+ charge. Use your knowledge of electron configurations to explain this observation.
(Essay)
4.8/5  (39)
(39)
Which arrangement is in the correct order of decreasing radii?
(Multiple Choice)
4.8/5  (46)
(46)
If each of the following metals is exposed to light with a wavelength of 240 nm, which will emit photoelectrons with the greatest kinetic energy?
(Multiple Choice)
4.7/5  (36)
(36)
What color will a blue object appear when it is seen through a filter with the absorption spectrum shown below? 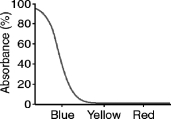
(Multiple Choice)
4.9/5  (38)
(38)
The ion of which element is most likely to have a charge of +2?
(Multiple Choice)
4.8/5  (39)
(39)
Which of the following orbitals is the lowest in energy (assuming they are all in the same shell)?
(Multiple Choice)
4.8/5  (42)
(42)
Based on the following graph, which metal will emit the highest energy photoelectron when a photon with a wavelength of 650 nm is incident on the metal? 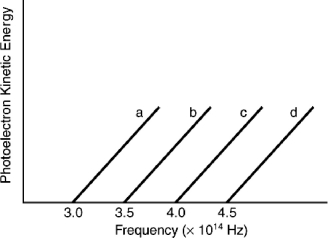
(Multiple Choice)
4.9/5  (32)
(32)
Showing 121 - 140 of 143
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)