Exam 7: A Quantum Model of Atoms: Waves and Particles
Exam 1: Matter and Energy: The Origin of the Universe99 Questions
Exam 2: Atoms, Ions, and Molecules: Matter Starts Here131 Questions
Exam 3: Stoichiometry: Mass, Formulas, and Reactions133 Questions
Exam 4: Solution Chemistry: The Hydrosphere126 Questions
Exam 5: Thermochemistry: Energy Changes in Reactions132 Questions
Exam 6: Properties of Gases: the Air We Breathe138 Questions
Exam 7: A Quantum Model of Atoms: Waves and Particles143 Questions
Exam 8: Chemical Bonds: What Makes a Gas a Greenhouse Gas139 Questions
Exam 9: Molecular Geometry: Shape Determines Function136 Questions
Exam 10: Intermolecular Forces: The Uniqueness of Water140 Questions
Exam 11: Solutions: Properties and Behavior130 Questions
Exam 12: Solids: Structures and Applications144 Questions
Exam 13: Organic Chemistry: Fuels, Pharmaceuticals, Materials, and Life129 Questions
Exam 14: Chemical Kinetics: Reactions in the Air We Breathe164 Questions
Exam 15: Chemical Equilibrium: How Much Product Does a Reaction Really Make91 Questions
Exam 16: Acid-Base and Solubility Equilibria: Reactions in Soil and Water179 Questions
Exam 17: Metal Ions: Colorful and Essential144 Questions
Exam 18: Thermodynamics: Spontaneous and Nonspontaneous Reactions and Processes157 Questions
Exam 19: Electrochemistry: the Quest for Clean Energy143 Questions
Exam 20: Biochemistry: the Compounds of Life108 Questions
Exam 21: Nuclear Chemistry: Applications to Energy and Medicine144 Questions
Exam 22: Life and the Periodic Table95 Questions
Select questions type
Which of the following electrons will have the smallest de Broglie wavelength?
(Multiple Choice)
4.7/5  (37)
(37)
What is the energy (E, in J) of the photons emitted by an Ar+ laser with a wavelength of = 488 nm?
(Multiple Choice)
4.9/5  (42)
(42)
Consider the radial distribution shown below for an electron in an s atomic orbital where r is the distance of the electron from the nucleus. 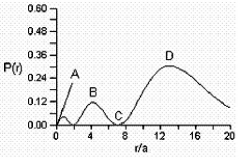 Identify: (1) where the atomic nucleus is located; (2) a radial node; (3) the most probable distance of the electron from the nucleus; and (4) the principle quantum number for this orbital.
Identify: (1) where the atomic nucleus is located; (2) a radial node; (3) the most probable distance of the electron from the nucleus; and (4) the principle quantum number for this orbital.
(Essay)
4.8/5  (37)
(37)
What is meant when two or more orbitals are said to be degenerate?
(Short Answer)
4.9/5  (42)
(42)
What are the principal and angular momentum quantum numbers for a 4f orbital?
(Multiple Choice)
5.0/5  (40)
(40)
Which statement A-D about the wave function for a single electron is not correct?
(Multiple Choice)
4.9/5  (41)
(41)
What is the kinetic energy of the photoelectrons emitted from a sodium surface (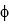 = 2.9 *10-19 J) when it is irradiated by photons with a wavelength of 350 nm?
= 2.9 *10-19 J) when it is irradiated by photons with a wavelength of 350 nm?
(Multiple Choice)
4.7/5  (32)
(32)
If cesium, which has a work function of 2.1 eV, is used in a photodetector, what will be the kinetic energy of electrons produced by green light (540 nm) incident on the detector?
(1 eV = 1.60 *10-19 J)
(Essay)
4.9/5  (38)
(38)
Based on the following graph, which metal will emit the highest energy photoelectron when a photon with a frequency of 3.9*1014 Hz is incident on the metal? 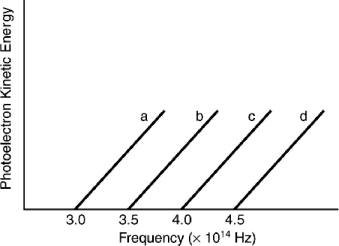
(Multiple Choice)
4.9/5  (33)
(33)
Indicate which of the following sources produces the lowest energy photons.
(Multiple Choice)
4.9/5  (36)
(36)
Which of the transitions in the hydrogen atom energy-level diagram shown here is not possible? 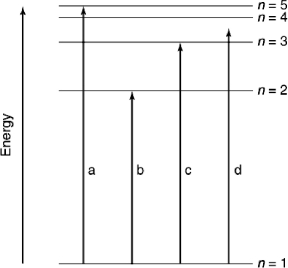
(Multiple Choice)
4.9/5  (35)
(35)
Indicate which metal requires the shortest wavelength photons to eject photoelectrons based on the following graph. 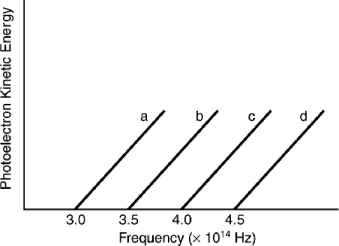
(Multiple Choice)
4.9/5  (30)
(30)
Which combination of quantum numbers is possible for an atom with five orbitals in one subshell?
(Multiple Choice)
4.9/5  (36)
(36)
Which of the following sources produces the highest energy photons?
(Multiple Choice)
4.7/5  (34)
(34)
Which arrangement is correct for increasing atomic radius?
(Multiple Choice)
4.7/5  (32)
(32)
Arrange the following elements in order of increasing size: 15P, 20Ca, 32Ge, 37Rb, 38Sr.
(Short Answer)
4.8/5  (35)
(35)
Which of the transitions in the hydrogen atom energy-level diagram shown here requires the longest wavelength photon? 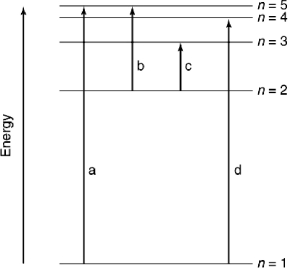
(Multiple Choice)
4.8/5  (41)
(41)
Showing 101 - 120 of 143
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)