Exam 19: Ionic Equilibria in Aqueous Systems
Exam 1: Keys to the Study of Chemistry68 Questions
Exam 2: The Components of Matter104 Questions
Exam 3: Stoichiometry of Formulas and Equations96 Questions
Exam 4: Three Major Classes of Chemical Reactions105 Questions
Exam 5: Gases and the Kinetic-Molecular Theory103 Questions
Exam 6: Thermochemistry: Energy Flow and Chemical Change79 Questions
Exam 7: Quantum Theory and Atomic Structure74 Questions
Exam 8: Electron Configuration and Chemical Periodicity81 Questions
Exam 9: Models of Chemical Bonding73 Questions
Exam 10: The Shapes of Molecules108 Questions
Exam 11: Theories of Covalent Bonding56 Questions
Exam 12: Intermolecular Forces: Liquids, Solids, and Phase Changes97 Questions
Exam 13: The Properties of Mixtures: Solutions and Colloids98 Questions
Exam 14: Periodic Patterns in the Main-Group Elements111 Questions
Exam 15: Organic Compounds and the Atomic Properties of Carbon113 Questions
Exam 16: Kinetics: Rates and Mechanisms of Chemical Reactions89 Questions
Exam 17: Equilibrium: the Extent of Chemical Reactions102 Questions
Exam 18: Acid-Base Equilibria106 Questions
Exam 19: Ionic Equilibria in Aqueous Systems115 Questions
Exam 20: Thermodynamics: Entropy, Free Energy, and the Direction of Chemical Reactions85 Questions
Exam 21: Electrochemistry: Chemical Change and Electrical Work102 Questions
Exam 22: The Elements in Nature and Industry56 Questions
Exam 23: The Transition Elements and Their Coordination Compounds92 Questions
Exam 24: Nuclear Reactions and Their Applications90 Questions
Select questions type
Calculate the solubility of magnesium sulfate, MgSO4, when placed into a 0.10 M MgCl2 solution. Ksp = 5.9 *10¯3
(Multiple Choice)
4.9/5  (36)
(36)
Which one of the following is the best representation of the titration curve which will be obtained in the titration of a weak base (0.10 mol L¯1) with HCl of the same concentration?
(Multiple Choice)
4.8/5  (37)
(37)
A 50.0-mL sample of 0.50 M HCl is titrated with 0.50 M NaOH. What is the pH of the solution after 28.0 mL of NaOH have been added to the acid?
(Multiple Choice)
4.8/5  (30)
(30)
A solution is prepared by mixing 50.0 mL of 0.50 M Cu(NO3)2 with 50.0 mL of 0.50 M Co(NO3)2. Sodium hydroxide is added to the mixture. Which hydroxide precipitates first and what concentration of hydroxide ions present in solution will accomplish the separation? Ksp = 2.2 *10¯20 for Cu(OH)2, Ksp = 1.3 *10¯15 for Co(OH)2
(Multiple Choice)
4.7/5  (29)
(29)
The concentration of the complex ion in each of following solutions is 1.00 M. In which of the solutions will the concentration of the uncomplexed metal ion be the greatest? 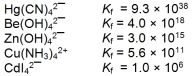
(Multiple Choice)
4.8/5  (42)
(42)
Citric acid has an acid dissociation constant of 8.4 *10¯4. It would be most effective for preparation of a buffer with a pH of:
(Multiple Choice)
4.8/5  (40)
(40)
What is the pH of a buffer that consists of 0.20 M NaH2PO4 and 0.40 M Na2HPO4? For NaH2PO4, Ka = 6.2*10¯8
(Multiple Choice)
4.8/5  (33)
(33)
The solubility of silver chromate is 0.0287 g/1.0 L of solution. What is the Ksp for Ag2CrO4?
(Multiple Choice)
4.9/5  (31)
(31)
Calculate the solubility of zinc hydroxide, Zn(OH)2, in 1.00 M NaOH. 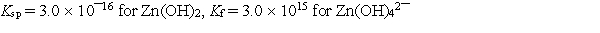
(Multiple Choice)
5.0/5  (40)
(40)
Increasing the concentrations of the components of a buffer solution will increase the buffer capacity.
(True/False)
4.9/5  (43)
(43)
What is the [H3O+] in a buffer that consists of 0.30 M HCOOH and 0.20 M HCOONa? For HCOOH, Ka = 1.7 * 10¯4
(Multiple Choice)
4.8/5  (39)
(39)
What volume of 0.500 M H2SO4 is needed to react completely with 20.0 mL of 0.400 M LiOH?
(Multiple Choice)
4.9/5  (40)
(40)
An acetate buffer has a pH of 4.40. Which of the following changes will cause the pH to decrease?
(Multiple Choice)
4.8/5  (42)
(42)
A buffer is prepared by adding 100 mL of 0.50 M sodium hydroxide to 100 mL of 0.75 M propanoic acid. Is this a buffer solution, and if so, what is its pH?
(Multiple Choice)
4.8/5  (44)
(44)
Use the following information to calculate the solubility product constant, Ksp, for CuCl. A saturated solution of CuCl in water was prepared and filtered. From the filtrate, 1.0 L was measured out into a beaker and evaporated to dryness. The solid CuCl residue recovered in the beaker was found to weigh 0.041g.
(Multiple Choice)
4.8/5  (39)
(39)
A solution is prepared by adding 500 mL of 0.3 M NaClO to 500 mL of 0.4 M HClO. What is the pH of this solution?
(Multiple Choice)
4.8/5  (39)
(39)
The end point in a titration is defined as the point when the indicator changes color.
(True/False)
4.9/5  (29)
(29)
What will be the effect of adding 0.5 mL of 0.1 M NaOH to 100 mL of an acetate buffer in which [CH3COOH] = [CH3COO¯] = 0.5 M?
(Multiple Choice)
4.8/5  (34)
(34)
The solubility of aluminum hydroxide in water ______________ when dilute nitric acid is added to it.
(Multiple Choice)
4.9/5  (34)
(34)
A 25.0-mL sample of 0.35 M HCOOH is titrated with 0.20 M KOH. What is the pH of the solution after 25.0 mL of KOH has been added to the acid? Ka = 1.77 * 10¯4
(Multiple Choice)
4.9/5  (35)
(35)
Showing 41 - 60 of 115
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)