Exam 16: Kinetics: Rates and Mechanisms of Chemical Reactions
Exam 1: Keys to the Study of Chemistry68 Questions
Exam 2: The Components of Matter104 Questions
Exam 3: Stoichiometry of Formulas and Equations96 Questions
Exam 4: Three Major Classes of Chemical Reactions105 Questions
Exam 5: Gases and the Kinetic-Molecular Theory103 Questions
Exam 6: Thermochemistry: Energy Flow and Chemical Change79 Questions
Exam 7: Quantum Theory and Atomic Structure74 Questions
Exam 8: Electron Configuration and Chemical Periodicity81 Questions
Exam 9: Models of Chemical Bonding73 Questions
Exam 10: The Shapes of Molecules108 Questions
Exam 11: Theories of Covalent Bonding56 Questions
Exam 12: Intermolecular Forces: Liquids, Solids, and Phase Changes97 Questions
Exam 13: The Properties of Mixtures: Solutions and Colloids98 Questions
Exam 14: Periodic Patterns in the Main-Group Elements111 Questions
Exam 15: Organic Compounds and the Atomic Properties of Carbon113 Questions
Exam 16: Kinetics: Rates and Mechanisms of Chemical Reactions89 Questions
Exam 17: Equilibrium: the Extent of Chemical Reactions102 Questions
Exam 18: Acid-Base Equilibria106 Questions
Exam 19: Ionic Equilibria in Aqueous Systems115 Questions
Exam 20: Thermodynamics: Entropy, Free Energy, and the Direction of Chemical Reactions85 Questions
Exam 21: Electrochemistry: Chemical Change and Electrical Work102 Questions
Exam 22: The Elements in Nature and Industry56 Questions
Exam 23: The Transition Elements and Their Coordination Compounds92 Questions
Exam 24: Nuclear Reactions and Their Applications90 Questions
Select questions type
In the gas phase at 500. C, cyclopropane reacts to form propene in a first-order reaction. The figure below shows the concentration of cyclopropane plotted versus time. Use the graph to calculate approximate values of
A) the rate of the reaction, 600. seconds after the start.
B) the half-life of the reaction, t1/2. 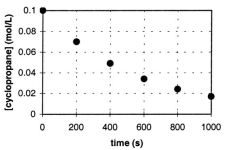
(Essay)
4.8/5  (34)
(34)
The rate constant for a reaction is 4.65 L mol¯1s¯1. What is the overall order of the reaction?
(Multiple Choice)
4.8/5  (39)
(39)
In the collision theory of reaction rates, the rate constant for a bimolecular reaction can be written as k = z · p · exp 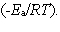 In one sentence each, clearly explain the physical meaning (interpretation) of the following three factors which appear in the above expression:
A) z
B) p
C) exp
In one sentence each, clearly explain the physical meaning (interpretation) of the following three factors which appear in the above expression:
A) z
B) p
C) exp 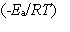
(Essay)
4.9/5  (41)
(41)
Consider the following mechanism for the oxidation of bromide ions by hydrogen peroxide in aqueous acid solution. 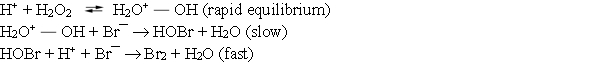 What is the overall reaction equation for this process?
What is the overall reaction equation for this process?
(Multiple Choice)
4.8/5  (33)
(33)
Carbon-14 is a radioactive isotope which decays with a half-life of 5730 years. What is the first-order rate constant for its decay, in units of years¯1?
(Multiple Choice)
4.8/5  (38)
(38)
The decomposition of dinitrogen pentaoxide to nitrogen dioxide and oxygen follows first-order kinetics and has an activation energy of 102 kJ/mol. By what factor will the fraction of collisions with energy greater than or equal to the activation energy increase if the reaction temperature goes from 30 C to 60 C?
(Multiple Choice)
4.9/5  (41)
(41)
Based on the initial rate data below, what is the value of the rate constant? 2NOBr(g) 2NO(g) + Br2(g) 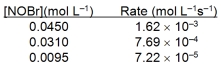
(Multiple Choice)
4.9/5  (32)
(32)
The rate constant for the reaction 3A 4B is 6.00 *10¯3 L mol¯1min¯1. How long will it take the concentration of A to drop from 0.75 M to 0.25 M?
(Multiple Choice)
4.8/5  (43)
(43)
Consider this reaction: 8A(g) + 5B(g) 8C(g) + 6D(g) If [C] is increasing at the rate of 4.0 mol L¯1s¯1, at what rate is [B] changing?
(Multiple Choice)
4.8/5  (37)
(37)
Showing 81 - 89 of 89
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)