Exam 16: Additional Aqueous Equilibria Chemistry and the Oceans
Exam 1: Matter and Energy an Atomic Perspective138 Questions
Exam 2: Atoms, Ions, and Molecules the Building Blocks of Matter143 Questions
Exam 3: Atomic Structure Explaining the Properties of Elements175 Questions
Exam 4: Chemical Bonding Understanding Climate Change182 Questions
Exam 5: Bonding Theories Explaining Molecular Geometry141 Questions
Exam 6: Intermolecular Forces Attractions Between Particles87 Questions
Exam 7: Stoichiometry Mass Relationships and Chemical Reactions140 Questions
Exam 8: Aqueous Solutions Chemistry of the Hydrosphere180 Questions
Exam 9: Thermochemistry Energy Changes in Chemical Reactions215 Questions
Exam 10: Properties of Gases the Air We Breathe164 Questions
Exam 11: Properties of Solutions Their Concentrations and Colligative Properties130 Questions
Exam 12: Thermodynamics Why Chemical Reactions Happen130 Questions
Exam 13: Chemical Kinetics Clearing the Air172 Questions
Exam 14: Chemical Equilibrium Equal but Opposite Reaction Rates119 Questions
Exam 15: Acid-Base Equilibria Proton Transfer in Biological Systems123 Questions
Exam 16: Additional Aqueous Equilibria Chemistry and the Oceans114 Questions
Exam 17: Electrochemistry the Quest for Clean Energy135 Questions
Exam 18: The Solid State a Particulate View170 Questions
Exam 19: Organic Chemistry Fuels, Pharmaceuticals, and Modern Materials145 Questions
Exam 20: Biochemistry the Compounds of Life153 Questions
Exam 21: Nuclear Chemistry the Risks and Benefits168 Questions
Exam 22: The Main Group Elements Life and the Periodic Table116 Questions
Exam 23: Transition Metals Biological and Medical Applications119 Questions
Select questions type
What would happen to the Ag+ and Cl- concentrations if NaCl(s) was added to a saturated solution of AgCl in water?
(Multiple Choice)
4.8/5  (34)
(34)
You are working on a project to recycle nickel and cadmium from old nickel-cadmium batteries that have an iron casing.The batteries are dissolved in aqueous nitric acid, producing a solution containing primarily Ni2+, Cd2+, and Fe3+ cations.One idea is to add sodium hydroxide to neutralize the acid and cause precipitation of Ni(OH)2, Cd(OH)2, and Fe(OH)3.Assume the concentration of each of the cations is 0.100 M before the sodium hydroxide is added.The pH increases as the sodium hydroxide is added.Which compound will precipitate first, and what is the pH at that point? 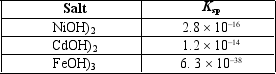
(Multiple Choice)
4.8/5  (41)
(41)
How many moles of sodium acetate must be added to 500.0 mL of 0.250 M acetic acid solution to produce a solution with a pH of 4.94? (The pKa of acetic acid is 4.74.)
(Multiple Choice)
4.8/5  (40)
(40)
Ksp for lead iodide is 8.5 *10-9 at 25°C.Will PbI2 precipitate if 250.0 mL of 0.0480 M NaI solution is added to 375.0 mL of 0.0388 M (PbNO3)2 solution?
(Essay)
4.7/5  (48)
(48)
Purveyors of salts from the Dead Sea advertise that it is healthy to bathe in a saturated solution of magnesium chloride (MgCl2, 95.21 g/mol, Ksp = 740).How much magnesium chloride would you have to purchase to make up 10.0 L of bath water saturated with magnesium chloride?
(Multiple Choice)
5.0/5  (36)
(36)
Consider the following aqueous equilibrium for a formic acid solution, HCOOH(aq) ⇄ H+(aq) + HCOO-(aq).Suppose solid sodium formate, NaHCOO, is added to the solution.Which of the following statements regarding the resulting solution is NOT correct? Ignore any volume changes.
(Multiple Choice)
4.8/5  (31)
(31)
A mining operation needs to separate silver and copper.You are to determine whether this separation can be accomplished by oxidizing the metals to Ag+ and Cu+, each one at a concentration of 0.15 M, and then precipitating one as the chloride salt without precipitating the other.What is the concentration of the Ag+ remaining in solution when CuCl begins to precipitate? (For AgCl, pKsp =9.74.and for CuCl, pKsp = 6.76.)
(Multiple Choice)
4.9/5  (42)
(42)
Ksp for silver sulfate is 1.2 * 10-5 at 25°C.What is the molar solubility of Ag2SO4 in
A) pure water and
B) 0.80 M sodium sulfate solution?
(Essay)
4.7/5  (33)
(33)
The solubility of PbBr2 is 0.427 g per 100.0 mL of solution at 25°C.Determine the value of the solubility product constant for this strong electrolyte.Lead(II) bromide does not react with water.
(Multiple Choice)
4.9/5  (42)
(42)
A solution with a pH of 9.100 is prepared using aqueous ammonia and solid ammonium chloride.What is the ratio of [NH3] to [NH4+] in the solution? The Kb of ammonia is 1.76 * 10-5.
(Multiple Choice)
5.0/5  (33)
(33)
Stalactites-the long, icicle-like formations that hang from the ceilings of caves-are formed from recrystallizing minerals like calcite (calcium carbonate, CaCO3, 100.09/mol).The Ksp of calcium carbonate is 4.5 * 10-9.What is the minimum volume of dripping water saturated with calcium carbonate that would be required to form a small stalactite that had a mass of 1.000 kg?
(Multiple Choice)
5.0/5  (38)
(38)
Stalactites-the long, icicle-like formations that hang from the ceilings of caves-are formed from recrystallizing minerals like calcite (calcium carbonate).The Ksp of calcium carbonate is 4.5 *10-9.What is the concentration of a saturated calcium carbonate solution?
(Multiple Choice)
4.7/5  (38)
(38)
The solubility product for Ag2S is written as _______, where s is the molar solubility.
(Multiple Choice)
4.9/5  (31)
(31)
What is the molar solubility of PbF2 in a buffer solution in which the [H+] concentration is roughly constant at 0.080 M? (Ksp of PbF2 is 3.2 *10-8; Ka of HF =6.8 * 10-4.)
(Multiple Choice)
4.9/5  (47)
(47)
Which of the statements below about an acid-base buffer solution is/are true?
I.It can be prepared by combining a strong acid with a salt of its conjugate base.
II.It can be prepared by combining a weak acid with a salt of its conjugate base.
III.It can be prepared by combining a weak base with its conjugate acid.
IV.The pH of a buffer solution does not change when the solution is diluted.
V.A buffer solution resists changes in its pH when an acid or base is added to it.
(Multiple Choice)
4.8/5  (37)
(37)
Acid-base indicators need to have very intense colors so that a very low concentration is visible.Could there be a problem in using too much indicator?
(Multiple Choice)
4.8/5  (33)
(33)
Showing 61 - 80 of 114
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)