Exam 16: Additional Aqueous Equilibria Chemistry and the Oceans
Exam 1: Matter and Energy an Atomic Perspective138 Questions
Exam 2: Atoms, Ions, and Molecules the Building Blocks of Matter143 Questions
Exam 3: Atomic Structure Explaining the Properties of Elements175 Questions
Exam 4: Chemical Bonding Understanding Climate Change182 Questions
Exam 5: Bonding Theories Explaining Molecular Geometry141 Questions
Exam 6: Intermolecular Forces Attractions Between Particles87 Questions
Exam 7: Stoichiometry Mass Relationships and Chemical Reactions140 Questions
Exam 8: Aqueous Solutions Chemistry of the Hydrosphere180 Questions
Exam 9: Thermochemistry Energy Changes in Chemical Reactions215 Questions
Exam 10: Properties of Gases the Air We Breathe164 Questions
Exam 11: Properties of Solutions Their Concentrations and Colligative Properties130 Questions
Exam 12: Thermodynamics Why Chemical Reactions Happen130 Questions
Exam 13: Chemical Kinetics Clearing the Air172 Questions
Exam 14: Chemical Equilibrium Equal but Opposite Reaction Rates119 Questions
Exam 15: Acid-Base Equilibria Proton Transfer in Biological Systems123 Questions
Exam 16: Additional Aqueous Equilibria Chemistry and the Oceans114 Questions
Exam 17: Electrochemistry the Quest for Clean Energy135 Questions
Exam 18: The Solid State a Particulate View170 Questions
Exam 19: Organic Chemistry Fuels, Pharmaceuticals, and Modern Materials145 Questions
Exam 20: Biochemistry the Compounds of Life153 Questions
Exam 21: Nuclear Chemistry the Risks and Benefits168 Questions
Exam 22: The Main Group Elements Life and the Periodic Table116 Questions
Exam 23: Transition Metals Biological and Medical Applications119 Questions
Select questions type
When sodium chloride is added to a saturated solution of lead(II) chloride, some of the lead(II) chloride precipitates.This phenomenon is called
(Multiple Choice)
4.8/5  (46)
(46)
A Lewis acid is any species capable of _______ an electron pair.
(Multiple Choice)
4.8/5  (37)
(37)
What are the characteristics of a pH indicator? I.It is usually a conjugate acid-base pair.
II)It has different characteristic colors in protonated and unprotonated forms.
III)It changes color when the pH is near its pKa.
(Multiple Choice)
4.9/5  (32)
(32)
Identify the Lewis base in the following reaction: SO2 (g) +H2 O(l) H2 SO3 (aq)
(Multiple Choice)
4.9/5  (48)
(48)
A 200.0 mL solution of 0.40 M ammonium chloride was titrated with 0.80M sodium hydroxide.What was the pH of the solution after 50.0 mL of the NaOH solution were added? The Kb of ammonia is 1.76 *10-5.
(Multiple Choice)
4.8/5  (37)
(37)
Ksp for lead(II) hydroxide is 1.4*10-20 at 25°C.What is the molar solubility of (PbOH)2 in
A) pure water and
B) a buffer solution with a pH= 11.50?
(Essay)
4.8/5  (27)
(27)
Which solution below would be the best choice for preparing a buffer with a pH=8.0?
(Multiple Choice)
4.8/5  (44)
(44)
What is the equilibrium concentration of Cu+(aq) in a solution that is initially 0.0200 M solution in CuNO3 and 0.450 M HCl(aq)?
Cu+(aq) + 3 Cl-(aq) fi CuCl 2-(aq) Kf * 5.0 *105
(Multiple Choice)
4.8/5  (40)
(40)
Research with biochemical systems commonly requires buffers because
(Multiple Choice)
4.9/5  (41)
(41)
The Yucca Mountain repository in Nevada, which is intended for the long-term storage of nuclear waste, has long been mired in controversy.One ongoing concern is whether the stainless-steel alloy containers could be corroded by salts such as calcium fluoride.If the calcium ion concentration in water inside Yucca Mountain is 1.25*10-3 M, what is the maximum possible concentration of fluoride ion in this water? Assume calcium fluoride Ksp = 2.2 *10-10 at the temperatures inside the nuclear waste depository.
(Multiple Choice)
4.7/5  (38)
(38)
What is the pH of a solution prepared by dissolving 4.50 g sodium dihydrogen borate (83.82g/mol) in 650 mL of a 0.0725 M boric acid? The Ka of boric acid is 5.4 *10-10.Ignore any volume change.
(Multiple Choice)
4.8/5  (36)
(36)
Glycolic acid, which is a monoprotic acid and a constituent in sugarcane, has a pKa of 3.9.A 25.0 mL solution of glycolic acid is titrated to the equivalence point with 35.8 mL of 0.020 M sodium hydroxide solution.What is the pH of the resulting solution at the equivalence point?
(Short Answer)
4.8/5  (32)
(32)
Suppose a 1.0 L solution containing 0.20 moles of morphine is titrated with 0.40 M HCl.The pH at the equivalence point is 4.543.What is the Kb of morphine?
(Multiple Choice)
4.7/5  (44)
(44)
The solubility product for Ag3PO4 is written as _______ , where s is the molar solubility.
(Multiple Choice)
4.9/5  (32)
(32)
At what point in the following titration curve for a weak acid being titrated with a strong base is the pH equal to the pKa of the acid? The x-axis scale goes from 0.0 mL to 20.0 mL.The sharp rise is at 10.0 mL. 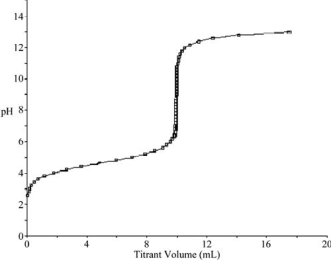
(Multiple Choice)
4.8/5  (45)
(45)
Identify the Lewis base in the following reaction: PH3 (aq) +H+(aq) PH4+ (aq)
(Multiple Choice)
4.7/5  (40)
(40)
A 25.00 mL sample of a phosphoric acid solution was titrated to completion with 37.04 mL of 0.1107 M sodium hydroxide.What was the concentration of the phosphoric acid?
(Multiple Choice)
4.9/5  (40)
(40)
Bromocresol green is yellow in its acidic form and blue in its basic form.When is it green?
(Multiple Choice)
4.8/5  (29)
(29)
Showing 41 - 60 of 114
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)