Exam 10: The Liquid and Solid States
Exam 1: Matter and Energy121 Questions
Exam 2: Atoms, Ions, and the Periodic Table143 Questions
Exam 3: Chemical Compounds113 Questions
Exam 4: Chemical Composition144 Questions
Exam 5: Chemical Reactions and Equations129 Questions
Exam 6: Quantities in Chemical Reactions133 Questions
Exam 7: Electron Structure of the Atom133 Questions
Exam 8: Chemical Bonding124 Questions
Exam 9: The Gaseous State121 Questions
Exam 10: The Liquid and Solid States118 Questions
Exam 11: Solutions119 Questions
Exam 12: Reaction Rates and Chemical Equilibrium110 Questions
Exam 13: Acids and Bases137 Questions
Exam 14: Oxidation-Reduction Reactions120 Questions
Exam 15: Nuclear Chemistry106 Questions
Exam 16: Organic Chemistry129 Questions
Exam 17: Biochemistry116 Questions
Select questions type
Calculate the amount of heat energy in kJ required to convert 45.0 g of ice at -15.5°C to steam at 124.0°C. (Cwater = 4.18 J/g°C; Cice = 2.03 J/g°C; Csteam = 2.02 J/g°C; molar heat of fusion of ice = 6.01 × 103 J/mol; molar heat of vaporization of liquid water = 4.07 × 104 J/mol)
(Short Answer)
4.8/5  (42)
(42)
Which choice correctly lists the intermolecular forces present in CH3Br?
(Multiple Choice)
4.9/5  (37)
(37)
The pictured change of state occurs at constant temperature even though heat is being added. Where does this change of state occur on the cooling curve? 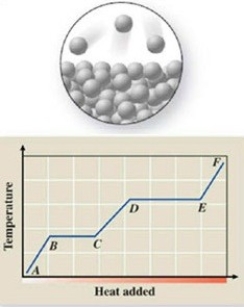
(Multiple Choice)
4.8/5  (42)
(42)
The diagram represents the physical state of a substance. Where does this physical state occur on the heating curve? 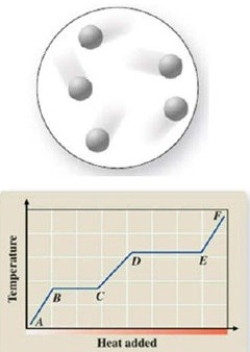
(Multiple Choice)
4.8/5  (27)
(27)
Rank the following substances in order of increasing boiling point: Cl2, Ar, Ne, Br2
(Multiple Choice)
4.8/5  (36)
(36)
Which of the following statements regarding intermolecular forces is incorrect?
(Multiple Choice)
4.9/5  (31)
(31)
Rank the following substances in order of increasing intermolecular forces: He, HF, F2, Ne
(Multiple Choice)
4.8/5  (40)
(40)
Three phases of water are shown in the figure. List the terms used to identify the phase changes indicated by the arrows. 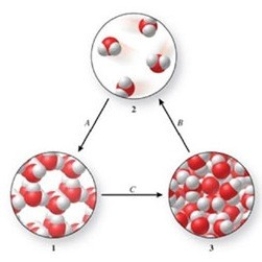
(Multiple Choice)
4.7/5  (29)
(29)
Which of the following statements regarding the liquid state is incorrect?
(Multiple Choice)
4.9/5  (30)
(30)
CH3OH boils at 64.7°C, and C2H6 boils at -88°C. Which types of intermolecular forces are responsible for the difference in their boiling points?
(Short Answer)
4.8/5  (33)
(33)
Which of the following substances is most likely to be a gas at room temperature and atmospheric pressure?
(Multiple Choice)
4.9/5  (37)
(37)
What phase change is occurring in the figure, and is it endothermic or exothermic? 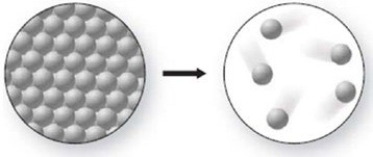
(Multiple Choice)
4.7/5  (44)
(44)
One would expect that since CO is polar, it has a higher boiling point than C6H14.
(True/False)
4.9/5  (30)
(30)
The substance shown in the figure must be a solid because you cannot see through it.  ©Jim Birk
©Jim Birk
(True/False)
4.9/5  (32)
(32)
Consider the melting point of the following solids. Which of these solids is most likely a molecular solid?
(Multiple Choice)
4.8/5  (37)
(37)
The molecular-level diagram of the substance shown must represent a liquid, since the atoms are not very close to one another. 
(True/False)
4.8/5  (26)
(26)
Calculate the amount of heat energy, in units of kilojoules, required to convert 50.0 g of liquid ethanol, CH3CH2OH, at 25.5°C to gaseous ethanol at 92.0°C. (Cethanol liquid = 2.44 J/g°C; Cethanol gas = 1.42 J/g°C; molar heat of vaporization of liquid ethanol = 3.86 × 104 J/mol. The boiling point of ethanol is 78.4°C and its molar mass is 46.07 g/mol.)
(Multiple Choice)
4.9/5  (41)
(41)
Identify the type of solid shown in the image. 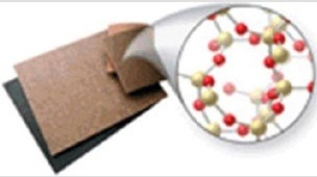 © C Squared Studios/Getty Images
© C Squared Studios/Getty Images
(Multiple Choice)
4.9/5  (37)
(37)
Showing 21 - 40 of 118
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)