Exam 10: The Liquid and Solid States
Exam 1: Matter and Energy121 Questions
Exam 2: Atoms, Ions, and the Periodic Table143 Questions
Exam 3: Chemical Compounds113 Questions
Exam 4: Chemical Composition144 Questions
Exam 5: Chemical Reactions and Equations129 Questions
Exam 6: Quantities in Chemical Reactions133 Questions
Exam 7: Electron Structure of the Atom133 Questions
Exam 8: Chemical Bonding124 Questions
Exam 9: The Gaseous State121 Questions
Exam 10: The Liquid and Solid States118 Questions
Exam 11: Solutions119 Questions
Exam 12: Reaction Rates and Chemical Equilibrium110 Questions
Exam 13: Acids and Bases137 Questions
Exam 14: Oxidation-Reduction Reactions120 Questions
Exam 15: Nuclear Chemistry106 Questions
Exam 16: Organic Chemistry129 Questions
Exam 17: Biochemistry116 Questions
Select questions type
What phase transition is occurring between points D and E on the heating curve? 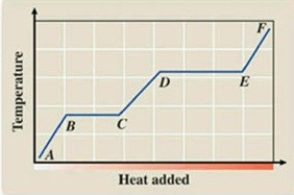
(Multiple Choice)
4.9/5  (33)
(33)
If there were no intermolecular forces, most matter would be in the gas phase at room temperature.
(True/False)
4.9/5  (36)
(36)
Which of the molecules in the figure has hydrogen bonding in the pure liquid state? 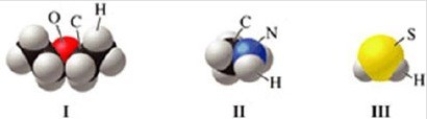
(Multiple Choice)
4.8/5  (43)
(43)
A solid substance has a very low melting point, is soft, and does not conduct heat or electricity. This substance is probably a(n)________ solid.
(Multiple Choice)
4.8/5  (41)
(41)
What causes water to have a high boiling point, high viscosity, and high surface tension, compared to other liquids?
(Short Answer)
4.7/5  (36)
(36)
Which of the following molecules experience dipole-dipole forces?
(Multiple Choice)
4.7/5  (31)
(31)
Rank the following substances in order of increasing boiling point: F2, Ne, He, Cl2
(Multiple Choice)
4.9/5  (36)
(36)
The diagram represents the physical state of a substance. Where does this physical state occur on the cooling curve? 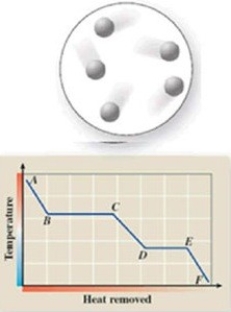
(Multiple Choice)
4.9/5  (40)
(40)
Which of the following is not one of the classifications into which solids can be organized?
(Multiple Choice)
4.7/5  (41)
(41)
Calculate the amount of heat energy in kJ required to convert 24.0 g of water at 25.5°C to steam at 124.0°C. (Cwater = 4.18 J/g°C; Csteam = 2.02 J/g°C; molar heat of vaporization of liquid water = 4.07 × 104 J/mol)
(Short Answer)
4.8/5  (30)
(30)
Calculate the amount of heat energy, in units of kilojoules, required to melt 75.0 g of ice at 0.0ºC. (molar heat of fusion of ice = 6.01 × 103 J/mol)
(Multiple Choice)
4.8/5  (34)
(34)
Explain why different liquids have different vapor pressures at a given temperature by filling in the blanks in the following statements: Different liquids have differing amounts of ________. Liquids with ________ intermolecular forces vaporize more easily at a given temperature, and thus will have a ________ vapor pressure than a liquid with ________ intermolecular forces.
(Short Answer)
4.9/5  (39)
(39)
The pictured change of state occurs at constant temperature even though heat is being removed. Where does this change of state occur on the cooling curve? 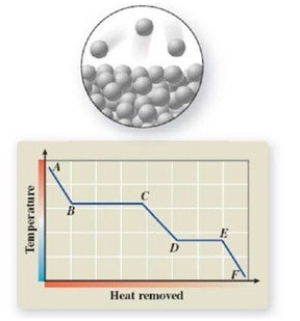
(Multiple Choice)
4.9/5  (30)
(30)
Select the pair of substances which has the one with the higher equilibrium vapor pressure at a given temperature listed first.
(Multiple Choice)
4.8/5  (49)
(49)
Showing 101 - 118 of 118
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)