Exam 10: The Liquid and Solid States
Exam 1: Matter and Energy121 Questions
Exam 2: Atoms, Ions, and the Periodic Table143 Questions
Exam 3: Chemical Compounds113 Questions
Exam 4: Chemical Composition144 Questions
Exam 5: Chemical Reactions and Equations129 Questions
Exam 6: Quantities in Chemical Reactions133 Questions
Exam 7: Electron Structure of the Atom133 Questions
Exam 8: Chemical Bonding124 Questions
Exam 9: The Gaseous State121 Questions
Exam 10: The Liquid and Solid States118 Questions
Exam 11: Solutions119 Questions
Exam 12: Reaction Rates and Chemical Equilibrium110 Questions
Exam 13: Acids and Bases137 Questions
Exam 14: Oxidation-Reduction Reactions120 Questions
Exam 15: Nuclear Chemistry106 Questions
Exam 16: Organic Chemistry129 Questions
Exam 17: Biochemistry116 Questions
Select questions type
Which of the following statements regarding the solid state is incorrect?
(Multiple Choice)
4.9/5  (32)
(32)
Which of the following statements regarding intermolecular forces is incorrect?
(Multiple Choice)
4.9/5  (38)
(38)
Which of the following has the highest vapor pressure at a given temperature?
(Multiple Choice)
5.0/5  (36)
(36)
A solid substance has a moderate melting point, is fairly soft, and is a nonconductor. This substance is probably a(n)________ solid.
(Multiple Choice)
4.9/5  (42)
(42)
Three phases of water are shown in the figure. List the terms used to identify the phase changes indicated by the arrows. 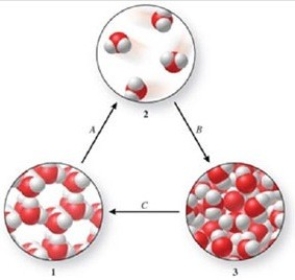
(Multiple Choice)
4.8/5  (44)
(44)
When liquid water is poured onto a flat plate of glass, it spreads out, while liquid mercury forms spherical beads under similar circumstances. Explain the difference in behavior of the two liquids by filling in the blanks in the following statements: The attractions between the water molecules and the glass are ________ than the attractions among the water molecules themselves, so the water spreads out. The attractions among the mercury atoms are ________ than the attractions between the mercury atoms and the glass, so it maintains a spherical shape.
(Essay)
4.8/5  (37)
(37)
Of the two molecules shown in the figure, one would expect that CO2 has a higher boiling point than NO2 because CO2 is linear and nonpolar. 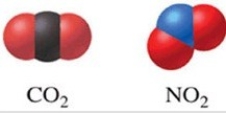
(True/False)
4.9/5  (37)
(37)
One would expect that water is more viscous than C2H6, due to differences in intermolecular forces.
(True/False)
4.8/5  (39)
(39)
Which of the following substances is most likely to be a gas at room temperature?
(Multiple Choice)
4.7/5  (35)
(35)
A solid substance has a high melting point, is hard, and is a good conductor of both heat and electricity. It is also malleable and ductile. This substance is probably a(n)________ solid.
(Multiple Choice)
4.8/5  (33)
(33)
Which of the following statements regarding the solid state is incorrect?
(Multiple Choice)
4.9/5  (43)
(43)
Which choice correctly lists the intermolecular forces present in CH3NH2?
(Multiple Choice)
5.0/5  (36)
(36)
Arrange the following substances in order of increasing boiling point: CH3OH, CH4, CH3CH2OH, HOCH2CH2OH
(Multiple Choice)
4.8/5  (30)
(30)
Calculate the amount of heat energy required to evaporate 55.0 g of water at 100.0ºC. (molar heat of vaporization of liquid water = 4.07 × 104 J/mol)
(Multiple Choice)
4.9/5  (40)
(40)
A solid substance has a very high melting point, is very hard, and is a nonconductor whether molten or solid. This substance is probably a(n)________ solid.
(Multiple Choice)
4.8/5  (38)
(38)
From the vapor pressure curve of acetone, it can be seen that the normal boiling point of acetone is about ________. 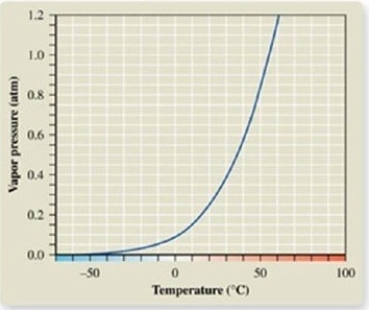
(Multiple Choice)
4.9/5  (42)
(42)
Showing 61 - 80 of 118
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)