Exam 16: Acids and Bases
Exam 1: Chemistry: the Central Science133 Questions
Exam 2: Atoms, Molecules, and Ions124 Questions
Exam 3: Stoichiometry: Ratios of Combination137 Questions
Exam 4: Reactions in Aqueous Solutions146 Questions
Exam 5: Thermochemistry141 Questions
Exam 6: Quantum Theory and the Electronic Structure of Atoms135 Questions
Exam 7: Electron Configuration and the Periodic Table120 Questions
Exam 8: Chemical Bonding I: Basic Concepts102 Questions
Exam 9: Chemical Bonding II: Molecular Geometry and Bonding Theories139 Questions
Exam 10: Gases137 Questions
Exam 11: Intermolecular Forces and the Physical Properties of Liquids and Solids137 Questions
Exam 12: Modern Materials108 Questions
Exam 13: Physical Properties of Solutions151 Questions
Exam 14: Chemical Kinetics132 Questions
Exam 15: Chemical Equilibrium146 Questions
Exam 16: Acids and Bases137 Questions
Exam 17: Acid-Base Equilibria and Solubility Equilibria133 Questions
Exam 18: Entropy, Free Energy, and Equilibrium107 Questions
Exam 19: Electrochemistry122 Questions
Exam 20: Nuclear Chemistry127 Questions
Exam 21: Environmental Chemistry135 Questions
Exam 22: Coordination Chemistry132 Questions
Exam 23: Metallurgy and the Chemistry of Metals152 Questions
Exam 24: Nonmetallic Elements and Their Compounds117 Questions
Exam 25: Organic Chemistry121 Questions
Select questions type
The pH of a Ba(OH)2 solution is 10.00. What is the H+ ion concentration of this solution?
(Multiple Choice)
4.8/5  (37)
(37)
After a substance acting as a strong base reacts, what remains of the base?
(Multiple Choice)
4.8/5  (44)
(44)
What is [OH-] for a solution at 25°C that has [H3O+] = 2.35 × 10-3 M?
(Multiple Choice)
4.8/5  (35)
(35)
What mass of sodium nitrite must be added to enough water to make 350.0 mL of a solution with pH = 8.40? [Ka(HNO2) = 5.6 × 10-4]
(Multiple Choice)
4.8/5  (49)
(49)
Below is a representation of an aqueous solution of a weak acid HA at equilibrium. (Each circle represents 1.0 ×10-3 mol of atoms, and the volume of the box is 1.0 L. Solvent water molecules are not shown for clarity.) 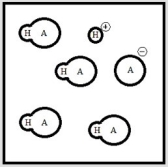 What is Ka of HA?
What is Ka of HA?
(Multiple Choice)
4.8/5  (34)
(34)
Calculate the pH of a 0.021 M NaCN solution. [Ka(HCN) = 4.9 × 10-10]
(Multiple Choice)
4.9/5  (36)
(36)
Find the pH of a 0.135 M aqueous solution of periodic acid (HIO4), for which Ka = 2.3 × 10-2.
(Multiple Choice)
4.9/5  (31)
(31)
Consider the weak bases below and their Kb values: C6H7O- Kb = 1.3 × 10-10
C2H5NH2 Kb = 5.6 × 10-4
C5H5N Kb = 1.7 ×10-9
Arrange the conjugate acids of these weak bases in order of increasing acid strength.
(Multiple Choice)
4.8/5  (41)
(41)
Which one of these salts will form a basic solution upon dissolving in water?
(Multiple Choice)
4.9/5  (28)
(28)
The most acidic oxides are formed from elements found in the ________ region of the periodic table.
(Multiple Choice)
4.7/5  (40)
(40)
The acid dissociation constant Ka equals 1.26 × 10-2 for HSO4- and is 5.6 × 10-10 for NH4+. Which statement about the following equilibrium is correct? HSO4-(aq) + NH3(aq)  SO42-(aq) + NH4+(aq)
SO42-(aq) + NH4+(aq)
(Multiple Choice)
4.7/5  (40)
(40)
Equal volumes of two solutions, one containing a strong acid at pH 2 and the other containing a strong base at pH 12, are mixed. Once equilibrium is achieved, what is the final pH of the combined solution?
(Multiple Choice)
4.8/5  (38)
(38)
If a strong acid such as HCl is diluted sufficiently with water, the pH will be higher than 7.
(True/False)
4.8/5  (45)
(45)
What is the pH of a 0.20 M solution of NH4Cl? [Kb(NH3) = 1.8 × 10-5]
(Multiple Choice)
4.7/5  (34)
(34)
Showing 61 - 80 of 137
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)