Exam 16: Acids and Bases
Exam 1: Chemistry: the Central Science133 Questions
Exam 2: Atoms, Molecules, and Ions124 Questions
Exam 3: Stoichiometry: Ratios of Combination137 Questions
Exam 4: Reactions in Aqueous Solutions146 Questions
Exam 5: Thermochemistry141 Questions
Exam 6: Quantum Theory and the Electronic Structure of Atoms135 Questions
Exam 7: Electron Configuration and the Periodic Table120 Questions
Exam 8: Chemical Bonding I: Basic Concepts102 Questions
Exam 9: Chemical Bonding II: Molecular Geometry and Bonding Theories139 Questions
Exam 10: Gases137 Questions
Exam 11: Intermolecular Forces and the Physical Properties of Liquids and Solids137 Questions
Exam 12: Modern Materials108 Questions
Exam 13: Physical Properties of Solutions151 Questions
Exam 14: Chemical Kinetics132 Questions
Exam 15: Chemical Equilibrium146 Questions
Exam 16: Acids and Bases137 Questions
Exam 17: Acid-Base Equilibria and Solubility Equilibria133 Questions
Exam 18: Entropy, Free Energy, and Equilibrium107 Questions
Exam 19: Electrochemistry122 Questions
Exam 20: Nuclear Chemistry127 Questions
Exam 21: Environmental Chemistry135 Questions
Exam 22: Coordination Chemistry132 Questions
Exam 23: Metallurgy and the Chemistry of Metals152 Questions
Exam 24: Nonmetallic Elements and Their Compounds117 Questions
Exam 25: Organic Chemistry121 Questions
Select questions type
What is the concentration of OH- in a 1.0 × 10-3 M Ba(OH)2 solution?
(Multiple Choice)
4.8/5  (40)
(40)
HCN is classified as a weak acid in water. What does this classification mean?
(Short Answer)
4.8/5  (36)
(36)
Farmers who raise cotton once used arsenic acid, H3AsO4, as a defoliant at harvest time. Arsenic acid is a polyprotic acid with Ka1 = 2.5 × 10-4, Ka2 = 5.6 × 10-8, and Ka3 = 3 × 10-13. What is the pH of a 0.500 M solution of arsenic acid?
(Multiple Choice)
4.9/5  (33)
(33)
Below is a representation of an aqueous solution of a weak acid HA at equilibrium. (Each circle represents 1.0 ×10-3 mol of atoms, and the volume of the box is 1.0 L. Solvent water molecules are not shown for clarity.) 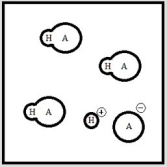 What is the pH of the solution?
What is the pH of the solution?
(Multiple Choice)
4.9/5  (33)
(33)
The following is the correct order for the acid strength for these oxoacids. HClO > HClO2 > HClO3 > HClO4
(True/False)
4.8/5  (40)
(40)
What is the name given to a substance that can act as a Brønsted acid or as a Brønsted base according to what it is reacting with?
(Multiple Choice)
4.9/5  (38)
(38)
Acid strength decreases in the series HI > HSO4-> HF > HCN. Which of these anions is the weakest base?
(Multiple Choice)
4.8/5  (38)
(38)
A(n) ________ can be added to a solution to increase the pH of the solution.
(Short Answer)
4.7/5  (41)
(41)
The most basic oxides are formed from elements found in the ________ region of the periodic table.
(Multiple Choice)
4.7/5  (31)
(31)
Which diagram best represents the products when equimolar amounts of HF(g) and NH3(g) react?
(Multiple Choice)
4.9/5  (35)
(35)
Below is a representation of pure liquid HX, an amphoteric substance, at equilibrium. (Each circle represents 1.0 ×10-3 mol of atoms, and the volume of the box is 1.0 L.) 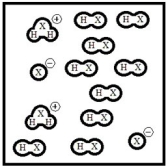 What is the autoionization constant for HX?
What is the autoionization constant for HX?
(Multiple Choice)
4.7/5  (37)
(37)
Showing 81 - 100 of 137
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)